A multicentre, efficacy and safety study of methotrexate to increase response rates in patients with uncontrolled gout receiving pegloticase (MIRROR): 12-month efficacy, safety, immunogenicity, and pharmacokinetic findings during long-term extension of an open-label study
- PMID: 36008814
- PMCID: PMC9404640
- DOI: 10.1186/s13075-022-02865-z
A multicentre, efficacy and safety study of methotrexate to increase response rates in patients with uncontrolled gout receiving pegloticase (MIRROR): 12-month efficacy, safety, immunogenicity, and pharmacokinetic findings during long-term extension of an open-label study
Abstract
Background: Publications suggest immunomodulation co-therapy improves responder rates in uncontrolled/refractory gout patients undergoing pegloticase treatment. The MIRROR open-label trial showed a 6-month pegloticase + methotrexate co-therapy responder rate of 79%, compared to an established 42% pegloticase monotherapy responder rate. Longer-term efficacy/safety data are presented here.
Methods: Uncontrolled gout patients (serum urate [SU] ≥ 6 mg/dL and SU ≥ 6 mg/dL despite urate-lowering therapy [ULT], ULT intolerance, or functionally-limiting tophi) were included. Patients with immunocompromised status, G6PD deficiency, severe kidney disease, or methotrexate contraindication were excluded. Oral methotrexate (15 mg/week) and folic acid (1 mg/day) were administered 4 weeks before and during pegloticase therapy. Twelve-month responder rate (SU < 6 mg/dL for ≥ 80% during month 12), 52-week change from baseline in SU, and extended safety were examined. Efficacy analyses were performed for patients receiving ≥ 1 pegloticase infusion. Pharmacokinetics (PK)/anti-drug antibodies (ADAs) were examined and related to efficacy/safety findings.
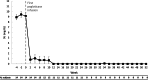
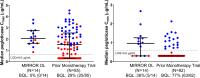
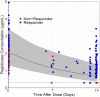
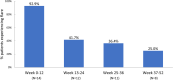
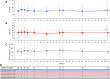
Results: Fourteen patients were included (all male, 49.3 ± 8.7 years, 13.8 ± 7.4-year gout history, pre-therapy SU 9.2 ± 2.5 mg/dL). Three patients were non-responders and discontinued study treatment before 24 weeks, one patient exited the study per protocol at 24 weeks (enrolled prior to treatment extension amendment), and 10 remained in the study through week 52. Of the 10, 8 completed 52 weeks of pegloticase + methotrexate and were 12-month responders. The remaining two discontinued pegloticase + methotrexate at week 24 (met treatment goals) and stayed in the study under observation (allopurinol prescribed at physicians' discretion); one remained a responder at 12 months. At 52 weeks, change from baseline in SU was - 8.2 ± 4.1 mg/dL (SU 1.1 ± 2.4 mg/dL, n = 10). Gout flares were common early in treatment but progressively decreased while on therapy (weeks 1-12, 13/14 [92.9%]; weeks 36-52, 2/8 [25.0%]). One patient recovered from sepsis (serious AE). Two non-responders developed high ADA titers; fewer patients had trough concentrations (Cmin) below the quantitation limit (BQL), and the median Cmin was higher (1.03 µg/mL vs. BQL) than pegloticase monotherapy trials.
Conclusions: Pegloticase + methotrexate co-therapy was well-tolerated over 12 months, with sustained SU lowering, progressive gout flare reduction, and no new safety concerns. Antibody/PK findings suggest methotrexate attenuates ADA formation, coincident with higher treatment response rates.
Trial registration: ClinicalTrials.gov, NCT03635957 . Registered on 17 August 2018.
Keywords: Gout; Methotrexate; Pegloticase; Tophi.
© 2022. The Author(s).
Conflict of interest statement
JKB has received research support from Horizon Therapeutics and Radius Health as a study site and principal investigator. He has received consulting/speaker fees > 10 k from Horizon Therapeutics, Celgene, Novartis, and AbbVie. JRPT has served as a consultant/advisory board member for BMS, Janssen, Lilly Pfizer, Sanofi-Genzyme, AbbVie, Aurinia, AstraZeneca, and Samumed/Biosplice. He has served as a speaker for AbbVie, Amgen, BMS, Janssen, Lilly, Pfizer, Sanofi/Genzyme, Aurinia, AstraZeneca, and GlaxoSmithKline. He has received research grants and support from AbbVie, Amgen, BMS, Boehringer Ingelheim, Genentech, Gilead, Horizon Therapeutics, Janssen, Lilly, Pfizer, Vorso, Samumed/Biosplice, Selecta, Exagen, CSL Behring, Organogenesis, SunPharma, DRL, and Emerald. RB declares that there are no competing interests. HMK has received research support from Horizon Therapeutics (study site/investigator), is an advisor and speaker for Horizon Therapeutics, and is an owner and chairman of the Board of Discus Analytics (JoinMan). KO, YS, BL, LZ, YX, and JC are employees of and own stock in Horizon Therapeutics. PMP and SR were employees of Horizon Therapeutics during the study and own stock in Horizon. MEW has received grants from Amgen, Bristol-Myers Squibb, Lilly, and Sanofi. He has received consulting fees greater than US $10,000 from Chemocentryx, Corona, and Genosco and less than US $10,000 from AbbVie, Amgen, Aclaris, Arena, Bayer, Bristol Meyer Squibb, Crescendo Myriad Genetics, GlaxoSmithKline, Gilead Sciences, Horizon Therapeutics, Johnson and Johnson, Eli Lilly, Novartis, Pfizer, Rani Therapeutics, Roche, Samsung, Scipher Medicine, Set Point, Tremeau, and XBiotech; he has stock options in Can-Fite BioPharma, Scipher Medicine, Inmedix, and Vorso and royalties from Elsevier as co-editor for the textbook
Figures
Similar articles
-
Improved joint and patient-reported health assessments with pegloticase plus methotrexate co-therapy in patients with uncontrolled gout: 12-month exploratory outcomes of the MIRROR open-label trial.Arthritis Res Ther. 2022 Dec 27;24(1):281. doi: 10.1186/s13075-022-02979-4. Arthritis Res Ther. 2022. PMID: 36575505 Free PMC article.
-
Community Practice Experiences with a Variety of Immunomodulatory Agents Co-Administered with Pegloticase for the Treatment of Uncontrolled Gout.Rheumatol Ther. 2022 Dec;9(6):1549-1558. doi: 10.1007/s40744-022-00492-3. Epub 2022 Sep 22. Rheumatol Ther. 2022. PMID: 36136270 Free PMC article.
-
Pegloticase in Combination With Methotrexate in Patients With Uncontrolled Gout: A Multicenter, Open-label Study (MIRROR).J Rheumatol. 2021 May;48(5):767-774. doi: 10.3899/jrheum.200460. Epub 2020 Sep 15. J Rheumatol. 2021. PMID: 32934137 Clinical Trial.
-
The effect of immunomodulators on the efficacy and tolerability of pegloticase: a systematic review.Semin Arthritis Rheum. 2021 Apr;51(2):347-352. doi: 10.1016/j.semarthrit.2021.01.005. Epub 2021 Jan 27. Semin Arthritis Rheum. 2021. PMID: 33601190
-
Recapture and improved outcome of pegloticase response with methotrexate-A report of two cases and review of the literature.Semin Arthritis Rheum. 2019 Aug;49(1):56-61. doi: 10.1016/j.semarthrit.2018.11.006. Epub 2018 Dec 4. Semin Arthritis Rheum. 2019. PMID: 30583886 Review.
Cited by
-
PEGylated porcine-human recombinant uricase: A novel fusion protein with improved efficacy and safety for the treatment of hyperuricemia and renal complications.Open Life Sci. 2024 Jan 23;19(1):20220799. doi: 10.1515/biol-2022-0799. eCollection 2024. Open Life Sci. 2024. PMID: 38283118 Free PMC article.
-
Advances in the use of nanotechnology for treating gout.Nanomedicine (Lond). 2025 Feb;20(4):355-369. doi: 10.1080/17435889.2025.2457315. Epub 2025 Jan 28. Nanomedicine (Lond). 2025. PMID: 39873132 Review.
-
Real-World Effectiveness of Pegloticase Associated With Use of Concomitant Immunomodulatory Therapy.Arthritis Care Res (Hoboken). 2024 Oct;76(10):1361-1370. doi: 10.1002/acr.25361. Epub 2024 Jun 23. Arthritis Care Res (Hoboken). 2024. PMID: 38719773
-
Treatment-emergent major adverse cardiovascular and thromboembolic events were infrequent during clinical trials of pegloticase.Rheumatology (Oxford). 2025 Jun 1;64(6):3328-3333. doi: 10.1093/rheumatology/keaf017. Rheumatology (Oxford). 2025. PMID: 39792028 Free PMC article.
-
The Singapore Experience With Uncontrolled Gout: Unmet Needs in the Management of Patients.Cureus. 2023 Mar 25;15(3):e36682. doi: 10.7759/cureus.36682. eCollection 2023 Mar. Cureus. 2023. PMID: 36987445 Free PMC article. Review.
References
-
- Choi HK, Ford ES, Li C, Curhan G. Prevalence of the metabolic syndrome in patients with gout: the third national health and nurtrition examination survey. Arthritis Care Res (Hoboken) 2007;57:109–115. - PubMed
-
- Zhao G, Huang L, Song M, Song Y. Baseline serum uric acid level as a predictor of cardiovascular disease related mortality and all-cause mortality: a meta-analysis of prospective studies. Atherosclerosis. 2013;231:61–68. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

