Association between low hemoglobin, clinical measures, and patient-reported outcomes in patients with rheumatoid arthritis: results from post hoc analyses of three phase III trials of sarilumab
- PMID: 36008838
- PMCID: PMC9404615
- DOI: 10.1186/s13075-022-02891-x
Association between low hemoglobin, clinical measures, and patient-reported outcomes in patients with rheumatoid arthritis: results from post hoc analyses of three phase III trials of sarilumab
Abstract
Background: Anemia is common in patients with rheumatoid arthritis (RA). Higher hemoglobin (Hb) levels may be associated with better clinical outcomes and patient-reported outcomes (PROs). To assess this hypothesis, we conducted two post hoc analyses in three sarilumab phase III studies: TARGET, MOBILITY, and MONARCH.
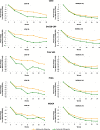
Methods: Pooled data from combination therapy from placebo-controlled MOBILITY (sarilumab + methotrexate) and TARGET (sarilumab + conventional synthetic disease-modifying antirheumatic drugs [csDMARDs]) and monotherapy data from active-controlled MONARCH (sarilumab vs. adalimumab) studies were included. Associations between Hb levels and clinical measures and PROs were assessed over 24 weeks. The mean changes from baseline in clinical outcomes and PROs (to week 24) and radiographic outcomes (to week 52) were evaluated between low and normal Hb levels (based on the World Health Organization [WHO] criteria).
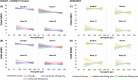
Results: From TARGET, MOBILITY, and MONARCH, 546, 1197, and 369 patients, respectively, were stratified according to Hb levels (low vs. normal). Over 24 weeks, higher Hb levels were found to be consistently associated with better clinical outcomes and PROs in combination therapy and monotherapy groups and were more pronounced among the patients treated with sarilumab than those treated with placebo and adalimumab. The mean change from baseline to week 24 in clinical efficacy measures and PROs was similar in patients with low vs. normal Hb at baseline. Differences between sarilumab and/or adalimumab, for all outcomes, were larger for low Hb subgroups. In MOBILITY, by week 52, the inhibition of progression of structural damage (assessed via Modified Total Sharp Score [mTSS]) was 84% (sarilumab 200 mg) and 68% (sarilumab 150 mg) vs. placebo in patients with low Hb and 97% (sarilumab 200 mg) and 68% (sarilumab 150 mg) vs. placebo in patients with normal Hb. Similar results were observed for other radiographic outcomes.
Conclusions: In these post hoc analyses, a consistent relationship was observed between higher Hb levels and better clinical outcomes and PROs in patients with RA. Irrespective of the baseline Hb levels, sarilumab treatment was associated with improvements in clinical measures and PROs over 24 weeks (improvements were more pronounced than those with adalimumab treatment) and mitigation of joint damage progression over 52 weeks.
Trial registration: ClinTrials.gov NCT01061736, NCT01709578, and NCT02332590.
Keywords: Anemia; Hemoglobin; MOBILITY; MONARCH; Patient-reported outcomes; Radiographic outcomes; Rheumatoid arthritis; Sarilumab; TARGET.
© 2022. The Author(s).
Conflict of interest statement
A. RR. has received honoraria for lectures and consultation from Sanofi, Abbvie, Lilly, Gilead, Roche, BMS, Boehringer, Novartis, Janssen, UCB, and Amgen.
D. F. has received grants and/or consulting fees from Actelion, Amgen, Bristol-Myers Squibb, Corbus, Galapagos, GlaxoSmithKline, Horizon, Kadmon, NIH, Novartis, Pfizer, Roche/Genentech, Talaris, and Sanofi Genzyme/Regeneron.
S. F. and A. P. are employees of Sanofi and may hold stock and/or stock options in the company.
V. B. has received consulting fees from Amgen; Bristol-Myers Squibb; Gilead; Pfizer; Regeneron Pharmaceuticals, Inc.; Sanofi Genzyme; and UCB, and her host institution has received research grants from Amgen, the Cedar Hill Foundation, and National Institutes of Health.
C. B. has received grants and/or consulting fees from AbbVie, Bristol-Myers Squibb, Janssen, Lilly, Pfizer, and Sanofi Genzyme/Regeneron.
C. CS. has received grants and/or consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, Gilead, Octopharma, Pfizer, and Sanofi Genzyme/Regeneron.
G.B. has received grant/research support, consulting fees, and/or speaker fees/honoraria from AbbVie, Lilly, Merck Sharp & Dohme, Pfizer, Roche, Sanofi Genzyme, and UCB.
Figures


References
-
- van Aken J, van Dongen H, le Cessie S, Allaart CF, Breedveld FC, Huizinga TW. Comparison of long term outcome of patients with rheumatoid arthritis presenting with undifferentiated arthritis or with rheumatoid arthritis: an observational cohort study. Ann Rheum Dis. 2006;65(1):20–25. doi: 10.1136/ard.2005.038471. - DOI - PMC - PubMed
-
- WHO. Chronic rheumatic conditions. Available from: https://www.who.int/chp/topics/rheumatic/en/. Accessed 11 Feb 2022.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

