Choroid and choriocapillaris changes in early-stage Parkinson's disease: a swept-source optical coherence tomography angiography-based cross-sectional study
- PMID: 36008844
- PMCID: PMC9404633
- DOI: 10.1186/s13195-022-01054-z
Choroid and choriocapillaris changes in early-stage Parkinson's disease: a swept-source optical coherence tomography angiography-based cross-sectional study
Abstract
Background: Parkinson's disease (PD) is one of the most common neurodegenerative diseases in the aging population. Previous literature has reported thinning of the retinal nerve fiber layer, ganglion cell layer, inner plexiform layer, and photoreceptor layer in PD patients. However, very few studies have used swept-source optical coherence tomography (SS-OCT) to study the choroid and choriocapillaris vascular changes in PD and their correlations with altered contrast sensitivity.
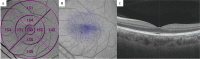
Methods: PD patients and controls were enrolled in the current study. We used a CSV-1000E instrument to assess contrast sensitivity and performed SS-OCT and SS-OCTA to measure outer retinal thickness, choroidal thickness, choriocapillaris flow density, choroidal vascular volume (CVV), and choroidal vascular index (CVI).
Results: One hundred eyes of 52 PD patients and 200 eyes of 100 healthy controls were recruited in the present study. Our study found remarkably impaired contrast sensitivity in PD patients (all P < 0.05). Significant thinning of the outer retinal layer and the choroid was appreciated in the PD group compared with the healthy controls (all P < 0.05). Choriocapillaris flow density, CVI, and CVV were significantly decreased in PD patients compared with healthy controls (all P < 0.05). Contrast sensitivity was weakly associated with outer retina thickness in the 3 mm circular area, with 3 cycles per degree being the most relevant (r = 0.535, P < 0.001).
Conclusion: Our study indicates that there is a significant decrease in contrast sensitivity, outer retina thickness, choriocapillaris flow density, CVI, and CVV in PD patients. This research has also identified a positive correlation between outer retina thickness and contrast sensitivity.
Keywords: Choriocapillaris; Optical coherence tomography angiography; Parkinson’s disease; Swept-source optical coherence tomography.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Retinal Flow Density Changes in Early-stage Parkinson's Disease Investigated by Swept-Source Optical Coherence Tomography Angiography.Curr Eye Res. 2021 Dec;46(12):1886-1891. doi: 10.1080/02713683.2021.1933054. Epub 2021 Aug 4. Curr Eye Res. 2021. PMID: 34348531
-
Retinal and Choroidal Changes in Patients with Parkinson's Disease Detected by Swept-Source Optical Coherence Tomography.Curr Eye Res. 2018 Jan;43(1):109-115. doi: 10.1080/02713683.2017.1370116. Epub 2017 Nov 7. Curr Eye Res. 2018. PMID: 29111842
-
Assessment of the outer retina and choroid in white matter lesions participants using swept-source optical coherence tomography.Brain Behav. 2021 Aug;11(8):e2240. doi: 10.1002/brb3.2240. Epub 2021 Jul 21. Brain Behav. 2021. PMID: 34291589 Free PMC article.
-
Retinal applications of swept source optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA).Prog Retin Eye Res. 2021 Sep;84:100951. doi: 10.1016/j.preteyeres.2021.100951. Epub 2021 Jan 28. Prog Retin Eye Res. 2021. PMID: 33516833 Review.
-
Update on visual function and choroidal-retinal thickness alterations in Parkinson's disease.Arch Soc Esp Oftalmol (Engl Ed). 2018 May;93(5):231-238. doi: 10.1016/j.oftal.2018.01.004. Epub 2018 Feb 14. Arch Soc Esp Oftalmol (Engl Ed). 2018. PMID: 29454631 Review. English, Spanish.
Cited by
-
Neuroretinal and microvascular retinal features in dementia with Lewy body assessed by optical coherence tomography angiography.Neurol Sci. 2025 Jan;46(1):185-194. doi: 10.1007/s10072-024-07683-6. Epub 2024 Aug 17. Neurol Sci. 2025. PMID: 39152330 Free PMC article.
-
Potential ocular indicators to distinguish posterior cortical atrophy and typical Alzheimer's disease: a cross-section study using optical coherence tomography angiography.Alzheimers Res Ther. 2024 Mar 25;16(1):64. doi: 10.1186/s13195-024-01431-w. Alzheimers Res Ther. 2024. PMID: 38528626 Free PMC article.
-
Assessment of Changes in Vascular Density in the Layers of the Eye in Patients With Parkinson's Disease.Ann Neurosci. 2024 Sep 3:09727531241259841. doi: 10.1177/09727531241259841. Online ahead of print. Ann Neurosci. 2024. PMID: 39544663 Free PMC article.
-
Optical Coherence Tomography Angiography: Revolutionizing Clinical Diagnostics and Treatment in Central Nervous System Disease.Aging Dis. 2024 Jan 20;16(1):77-114. doi: 10.14336/AD.2024.0112. Online ahead of print. Aging Dis. 2024. PMID: 38300645 Free PMC article. Review.
-
Keeping an eye on Parkinson's disease: color vision and outer retinal thickness as simple and non-invasive biomarkers.J Neurol. 2025 Apr 21;272(5):351. doi: 10.1007/s00415-025-13080-6. J Neurol. 2025. PMID: 40257599 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

