Detergent-Free Isolation of Membrane Proteins and Strategies to Study Them in a Near-Native Membrane Environment
- PMID: 36008970
- PMCID: PMC9406181
- DOI: 10.3390/biom12081076
Detergent-Free Isolation of Membrane Proteins and Strategies to Study Them in a Near-Native Membrane Environment
Abstract
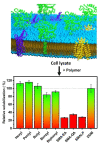
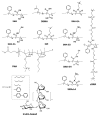
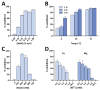
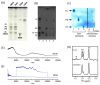
Atomic-resolution structural studies of membrane-associated proteins and peptides in a membrane environment are important to fully understand their biological function and the roles played by them in the pathology of many diseases. However, the complexity of the cell membrane has severely limited the application of commonly used biophysical and biochemical techniques. Recent advancements in NMR spectroscopy and cryoEM approaches and the development of novel membrane mimetics have overcome some of the major challenges in this area. For example, the development of a variety of lipid-nanodiscs has enabled stable reconstitution and structural and functional studies of membrane proteins. In particular, the ability of synthetic amphipathic polymers to isolate membrane proteins directly from the cell membrane, along with the associated membrane components such as lipids, without the use of a detergent, has opened new avenues to study the structure and function of membrane proteins using a variety of biophysical and biological approaches. This review article is focused on covering the various polymers and approaches developed and their applications for the functional reconstitution and structural investigation of membrane proteins. The unique advantages and limitations of the use of synthetic polymers are also discussed.
Keywords: NMR; cryoEM; detergent-free membrane protein isolation; ionic and non-ionic polymers; lipid-nanodisc; membrane protein stability and structure.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Microfluidic diffusional sizing probes lipid nanodiscs formation.Biochim Biophys Acta Biomembr. 2020 Jun 1;1862(6):183215. doi: 10.1016/j.bbamem.2020.183215. Epub 2020 Feb 20. Biochim Biophys Acta Biomembr. 2020. PMID: 32061645
-
Interfacing Membrane Mimetics with Mass Spectrometry.Acc Chem Res. 2016 Nov 15;49(11):2459-2467. doi: 10.1021/acs.accounts.6b00379. Epub 2016 Oct 13. Acc Chem Res. 2016. PMID: 27736086 Free PMC article.
-
Enhancing the stability and homogeneity of non-ionic polymer nanodiscs by tuning electrostatic interactions.J Colloid Interface Sci. 2023 Mar 15;634:887-896. doi: 10.1016/j.jcis.2022.12.112. Epub 2022 Dec 21. J Colloid Interface Sci. 2023. PMID: 36566634 Free PMC article.
-
Polymer nanodiscs: Advantages and limitations.Chem Phys Lipids. 2019 Mar;219:45-49. doi: 10.1016/j.chemphyslip.2019.01.010. Epub 2019 Jan 29. Chem Phys Lipids. 2019. PMID: 30707909 Free PMC article. Review.
-
Native-like environments afford novel mechanistic insights into membrane proteins.Trends Biochem Sci. 2022 Jul;47(7):561-569. doi: 10.1016/j.tibs.2022.02.008. Epub 2022 Mar 21. Trends Biochem Sci. 2022. PMID: 35331611 Free PMC article. Review.
Cited by
-
Fabrication of membrane proteins in the form of native cell membrane nanoparticles using novel membrane active polymers.Nanoscale Adv. 2023 Oct 9;5(21):5932-5940. doi: 10.1039/d3na00381g. eCollection 2023 Oct 24. Nanoscale Adv. 2023. PMID: 37881706 Free PMC article.
-
Non-Ionic Inulin-Based Polymer Nanodiscs Enable Functional Reconstitution of a Redox Complex Composed of Oppositely Charged CYP450 and CPR in a Lipid Bilayer Membrane.Anal Chem. 2022 Aug 30;94(34):11908-11915. doi: 10.1021/acs.analchem.2c02489. Epub 2022 Aug 17. Anal Chem. 2022. PMID: 35977417 Free PMC article.
-
Characterization of nanodisc-forming peptides for membrane protein studies.J Colloid Interface Sci. 2024 Jan;653(Pt B):1402-1414. doi: 10.1016/j.jcis.2023.09.162. Epub 2023 Sep 29. J Colloid Interface Sci. 2024. PMID: 37801850 Free PMC article.
-
Fab-Induced Stabilization of an Ion Channel Receptor Enables Mechanistic Characterization of Small-Molecule Therapeutics.Anal Chem. 2025 Mar 11;97(9):5102-5108. doi: 10.1021/acs.analchem.4c06339. Epub 2025 Feb 24. Anal Chem. 2025. PMID: 39993138
-
The application of nanodiscs in membrane protein drug discovery & development and drug delivery.Front Chem. 2024 Sep 18;12:1444801. doi: 10.3389/fchem.2024.1444801. eCollection 2024. Front Chem. 2024. PMID: 39359422 Free PMC article. Review.
References
-
- Bernaudat F., Frelet-Barrand A., Pochon N., Dementin S., Hivin P., Boutigny S., Rioux J.-B., Salvi D., Seigneurin-Berny D., Richaud P., et al. Heterologous expression of membrane proteins: Choosing the appropriate host. PLoS ONE. 2011;6:e29191. doi: 10.1371/journal.pone.0029191. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

