Interaction of the Emerging Mycotoxins Beauvericin, Cyclopiazonic Acid, and Sterigmatocystin with Human Serum Albumin
- PMID: 36009000
- PMCID: PMC9406214
- DOI: 10.3390/biom12081106
Interaction of the Emerging Mycotoxins Beauvericin, Cyclopiazonic Acid, and Sterigmatocystin with Human Serum Albumin
Abstract
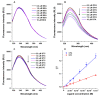
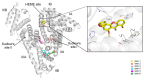
Beauvericin (BEA), cyclopiazonic acid (CPA), and sterigmatocystin (STC) are emerging mycotoxins. They appear as contaminants in food and animal feed, leading to economic losses and health risks. Human serum albumin (HSA) forms stable complexes with certain mycotoxins, including ochratoxins, alternariol, citrinin, and zearalenone. HSA binding can influence the toxicokinetics of xenobiotics, and albumin can also be considered and applied as a relatively cheap affinity protein. Therefore, we examined the potential interactions of BEA, CPA, and STC with HSA employing fluorescence spectroscopy, ultracentrifugation, ultrafiltration, and molecular modeling. Spectroscopic and ultracentrifugation studies demonstrated the formation of low-affinity BEA-HSA (Ka ≈ 103 L/mol) and moderately strong CPA-HSA and STC-HSA complexes (Ka ≈ 104 L/mol). In ultrafiltration experiments, CPA slightly displaced each site marker (warfarin, naproxen, and camptothecin) tested, while BEA and STC did not affect significantly the albumin binding of these drugs. Modeling studies suggest that CPA occupies Sudlow's site I, while STC binds to the Heme site (FA1) on HSA. Considering the interactions of CPA with the site markers, the CPA-HSA interaction may have toxicological importance.
Keywords: albumin–ligand interaction; beauvericin; cyclopiazonic acid; human serum albumin; sterigmatocystin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Tedjiotsop Feudjio F., Dornetshuber R., Lemmens M., Hoffmann O., Lemmens-Gruber R., Berger W. Beauvericin and Enniatin: Emerging Toxins and/or Remedies? World Mycotoxin J. 2010;3:415–430. doi: 10.3920/WMJ2010.1245. - DOI
-
- Santini A., Meca G., Uhlig S., Ritieni A. Fusaproliferin, Beauvericin and Enniatins: Occurrence in Food—A Review. World Mycotoxin J. 2012;5:71–81. doi: 10.3920/WMJ2011.1331. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

