Translocation of Distinct Alpha Synuclein Species from the Nucleus to Neuronal Processes during Neuronal Differentiation
- PMID: 36009004
- PMCID: PMC9406079
- DOI: 10.3390/biom12081108
Translocation of Distinct Alpha Synuclein Species from the Nucleus to Neuronal Processes during Neuronal Differentiation
Abstract
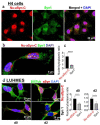
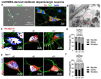
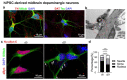
Alpha synuclein (aSyn) and its aggregation are crucial for the neurodegeneration of Parkinson's disease (PD). aSyn was initially described in the nucleus and presynaptic nerve terminals. However, the biology of nuclear aSyn and the link of aSyn between subcellular compartments are less understood. Current knowledge suggests the existence of various aSyn species with distinct structural and biochemical properties. Here, we identified a C-terminal-targeting aSyn antibody (Nu-aSyn-C), which has a high immunoaffinity towards aSyn in the nucleus. Comparing the Nu-aSyn-C antibody to aSyn antibodies developed against phosphorylated or aggregated forms, we observed that nuclear aSyn differs from cytosolic aSyn by an increased phosphorylation and assembly level in proliferating cells. Employing Nu-aSyn-C, we characterized aSyn distribution during neuronal differentiation in midbrain dopaminergic neurons (mDANs) derived from human-induced pluripotent stem cells (hiPSCs) and Lund human mesencephalic cells, and in primary rat hippocampal neurons. We detected a specific translocation pattern of aSyn during neuronal differentiation from the nucleus to the soma and finally to neuronal processes. Interestingly, a remarkable shift of Nu-aSyn-C-positive species towards neurites was detected in hiPSC mDANs from a PD patient carrying aSyn gene duplication. Together, our results reveal distinct nuclear and cytosolic aSyn species that redistribute during neuronal differentiation-a process that is altered in PD-derived neurons.
Keywords: Parkinson’s disease; SNCA duplication; alpha synuclein; nuclear alpha synuclein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Vimentin network dysregulation mediates neurite deficits in SNCA duplication Parkinson's patient-derived midbrain neurons.Sci Adv. 2025 Jun 6;11(23):eadq2742. doi: 10.1126/sciadv.adq2742. Epub 2025 Jun 6. Sci Adv. 2025. PMID: 40479066 Free PMC article.
-
Interaction of Alpha Synuclein and Microtubule Organization Is Linked to Impaired Neuritic Integrity in Parkinson's Patient-Derived Neuronal Cells.Int J Mol Sci. 2022 Feb 5;23(3):1812. doi: 10.3390/ijms23031812. Int J Mol Sci. 2022. PMID: 35163733 Free PMC article.
-
Demonstration of brain region-specific neuronal vulnerability in human iPSC-based model of familial Parkinson's disease.Hum Mol Genet. 2020 May 8;29(7):1180-1191. doi: 10.1093/hmg/ddaa039. Hum Mol Genet. 2020. PMID: 32160287 Free PMC article.
-
Novel Immunotherapeutic Approaches to Target Alpha-Synuclein and Related Neuroinflammation in Parkinson's Disease.Cells. 2019 Jan 31;8(2):105. doi: 10.3390/cells8020105. Cells. 2019. PMID: 30708997 Free PMC article. Review.
-
Oxidative and nitrative alpha-synuclein modifications and proteostatic stress: implications for disease mechanisms and interventions in synucleinopathies.J Neurochem. 2013 May;125(4):491-511. doi: 10.1111/jnc.12226. Epub 2013 Mar 19. J Neurochem. 2013. PMID: 23452040 Review.
Cited by
-
NRN1 interacts with Notch to increase oncogenic STAT3 signaling in melanoma.Cell Commun Signal. 2024 May 6;22(1):256. doi: 10.1186/s12964-024-01632-8. Cell Commun Signal. 2024. PMID: 38705997 Free PMC article.
-
Unraveling the Complex Interplay between Alpha-Synuclein and Epigenetic Modification.Int J Mol Sci. 2023 Apr 2;24(7):6645. doi: 10.3390/ijms24076645. Int J Mol Sci. 2023. PMID: 37047616 Free PMC article. Review.
-
Vimentin network dysregulation mediates neurite deficits in SNCA duplication Parkinson's patient-derived midbrain neurons.Sci Adv. 2025 Jun 6;11(23):eadq2742. doi: 10.1126/sciadv.adq2742. Epub 2025 Jun 6. Sci Adv. 2025. PMID: 40479066 Free PMC article.
-
Nuclear Alpha-Synuclein in Parkinson's Disease and the Malignant Transformation in Melanoma.Neurol Res Int. 2025 Jan 6;2025:1119424. doi: 10.1155/nri/1119424. eCollection 2025. Neurol Res Int. 2025. PMID: 39816956 Free PMC article.
-
Nuclear α-Synuclein-Derived Cytotoxic Effect via Altered Ribosomal RNA Processing in Primary Mouse Embryonic Fibroblasts.Int J Mol Sci. 2023 Jan 21;24(3):2132. doi: 10.3390/ijms24032132. Int J Mol Sci. 2023. PMID: 36768455 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

