Structure-Activity Relationship of the Dimeric and Oligomeric Forms of a Cytotoxic Biotherapeutic Based on Diphtheria Toxin
- PMID: 36009005
- PMCID: PMC9406121
- DOI: 10.3390/biom12081111
Structure-Activity Relationship of the Dimeric and Oligomeric Forms of a Cytotoxic Biotherapeutic Based on Diphtheria Toxin
Abstract
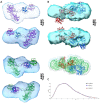
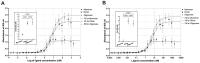
Protein aggregation is a well-recognized problem in industrial preparation, including biotherapeutics. These low-energy states constantly compete with a native-like conformation, which is more pronounced in the case of macromolecules of low stability in the solution. A better understanding of the structure and function of such aggregates is generally required for the more rational development of therapeutic proteins, including single-chain fusion cytotoxins to target specific receptors on cancer cells. Here, we identified and purified such particles as side products of the renaturation process of the single-chain fusion cytotoxin, composed of two diphtheria toxin (DT) domains and interleukin 13 (IL-13), and applied various experimental techniques to comprehensively understand their molecular architecture and function. Importantly, we distinguished soluble purified dimeric and fractionated oligomeric particles from aggregates. The oligomers are polydisperse and multimodal, with a distribution favoring lower and even stoichiometries, suggesting they are composed of dimeric building units. Importantly, all these oligomeric particles and the monomer are cystine-dependent as their innate disulfide bonds have structural and functional roles. Their reduction triggers aggregation. Presumably the dimer and lower oligomers represent the metastable state, retaining the native disulfide bond. Although significantly reduced in contrast to the monomer, they preserve some fraction of bioactivity, manifested by their IL-13RA2 receptor affinity and selective cytotoxic potency towards the U-251 glioblastoma cell line. These molecular assemblies probably preserve structural integrity and native-like fold, at least to some extent. As our study demonstrated, the dimeric and oligomeric cytotoxin may be an exciting model protein, introducing a new understanding of its monomeric counterpart's molecular characteristics.
Keywords: IL-13; LC/MS; MALS; SAXS; biotherapeutics; cytotoxin; diphtheria toxin; disulfide bond; inclusion bodies; protein oligomerization; refolding.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures








Similar articles
-
Dimeric form of diphtheria toxin: purification and characterization.Biochemistry. 1986 May 6;25(9):2425-30. doi: 10.1021/bi00357a019. Biochemistry. 1986. PMID: 3718959
-
Refined structure of monomeric diphtheria toxin at 2.3 A resolution.Protein Sci. 1994 Sep;3(9):1464-75. doi: 10.1002/pro.5560030912. Protein Sci. 1994. PMID: 7833808 Free PMC article.
-
Domain swapping: entangling alliances between proteins.Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3127-31. doi: 10.1073/pnas.91.8.3127. Proc Natl Acad Sci U S A. 1994. PMID: 8159715 Free PMC article.
-
Targeting diphtheria toxin to growth factor receptors.Semin Cancer Biol. 1995 Oct;6(5):259-67. doi: 10.1006/scbi.1995.0034. Semin Cancer Biol. 1995. PMID: 8562903 Review.
-
Diphtheria-related peptide hormone gene fusions: a molecular genetic approach to chimeric toxin development.Cancer Treat Res. 1988;37:123-40. doi: 10.1007/978-1-4613-1083-9_9. Cancer Treat Res. 1988. PMID: 2908622 Review.
Cited by
-
Targeting Neurological Disorders with Stilbenes: Bridging the Preclinical-Clinical Gap.Int J Biol Sci. 2024 Oct 7;20(14):5474-5494. doi: 10.7150/ijbs.102032. eCollection 2024. Int J Biol Sci. 2024. PMID: 39494329 Free PMC article. Review.
References
-
- Garashchenko B.L., Korsakova V.A., Yakovlev R.Y. Radiopharmaceuticals Based on Alpha Emitters: Preparation, Properties, and Application. Phys. At. Nucl. 2018;81:1515–1525. doi: 10.1134/S1063778818100071. - DOI
-
- Pagliaro L.C., Liu B., Munker R., Andreeff M., Freireich E.J., Scheinberg D.A., Rosenblum M.G. Humanized M195 monoclonal antibody conjugated to recombinant gelonin: An anti-CD33 immunotoxin with antileukemic activity. Clin. Cancer Res. 1998;4:1971–1976. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

