Race for the Cure: From the Oldest to the Newest Monoclonal Antibodies for Multiple Myeloma Treatment
- PMID: 36009041
- PMCID: PMC9405888
- DOI: 10.3390/biom12081146
Race for the Cure: From the Oldest to the Newest Monoclonal Antibodies for Multiple Myeloma Treatment
Abstract
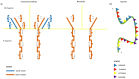
Multiple myeloma is characterized by a wide clinical heterogeneity due to an intricate network of interactions between bone marrow-resident clonal plasma cells and the microenvironment. Over the last years, dramatic improvement in the understanding of these pathways led to the introduction of novel drugs with immune-mediated mechanisms of action. Some of these compounds, such as the anti-cd38 daratumumab and isatuximab, the anti-slamf-7 elotuzumab, and the antibody-drug conjugate belantamab-mafodotin, have been tested in large clinical trials and have now fully entered the real-life management. The bispecific T-cell engagers are under investigation with promising results, and other satisfactory data is expected from the application of nanotechnologies. The perfect timing to introduce these drugs in the sequence of treatment and their adverse events represent new challenges to be addressed, and further experience is required to improve their use.
Keywords: immune therapy; monoclonal antibodies; multiple myeloma; nanobodies.
Conflict of interest statement
G.L. and F.F. have nothing to declare. M.T.P. served as a consultant or on an advisory board for and received honoraria from Janssen-Cilag, Celgene, Bristol-Myers Squibb, Amgen, Takeda, and Sanofi.
Figures

References
-
- Usmani S.Z., Hoering A., Cavo M., Miguel J.S., Goldschimdt H., Hajek R., Turesson I., Lahuerta J.J., Attal M., Barlogie B., et al. Clinical predictors of long-term survival in newly diagnosed transplant eligible multiple myeloma—An IMWG Research Project. Blood Cancer J. 2018;8:123. doi: 10.1038/s41408-018-0155-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

