The Beneficial Effects of Ultramicronized Palmitoylethanolamide in the Management of Neuropathic Pain and Associated Mood Disorders Induced by Paclitaxel in Mice
- PMID: 36009049
- PMCID: PMC9406031
- DOI: 10.3390/biom12081155
The Beneficial Effects of Ultramicronized Palmitoylethanolamide in the Management of Neuropathic Pain and Associated Mood Disorders Induced by Paclitaxel in Mice
Abstract
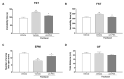
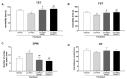
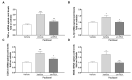
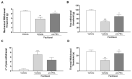
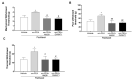
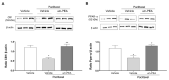
Chemotherapy-induced peripheral neuropathy (CIPN) is a common complication of antineoplastic drugs, particularly paclitaxel (PTX). It can affect the quality of patients' lives and increase the risk of developing mood disorders. Although several drugs are recommended, they yielded inconclusive results in clinical trials. The aim of the present work is to investigate whether the palmitoylethanolamide (PEA) would reduce PTX-induced CIPN and associated mood disorders. Moreover, the role PPAR-α and the endocannabinoid system will also be investigated. CIPN was induced by intraperitoneally injection of PTX (8 mg/kg) every other day for a week. PEA, 30 mg/kg, was orally administrated in a bioavailable form (i.e., ultramicronized PEA, um-PEA) one hour after the last PTX injection, for 7 days. In the antagonism experiments, AM281 (1 mg/kg) and GW6471 (2 mg/kg) were administrated 30 min before um-PEA. Our results demonstrated that um-PEA reduced the development of hypersensitivity with the effect being associated with the reduction in spinal and hippocampal pro-inflammatory cytokines, as well as antidepressive and anxiolytic effects. Moreover, the PPAR-α and CB1 receptor antagonists blocked the behavioral and antinociceptive effects of um-PEA. Our findings suggest that um-PEA is a promising adjunct in CIPN and associated mood disorders through the activation of PPAR-α, which influences the endocannabinoid system.
Keywords: PPAR-α; behavior; cannabinoids; inflammation; neuropathic pain; paclitaxel; um-PEA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Palmitoylethanolamide Reverses Paclitaxel-Induced Allodynia in Mice.J Pharmacol Exp Ther. 2016 Nov;359(2):310-318. doi: 10.1124/jpet.116.236182. Epub 2016 Sep 8. J Pharmacol Exp Ther. 2016. PMID: 27608657 Free PMC article.
-
Protective Effects of Ultramicronized Palmitoylethanolamide (PEA-um) in Myocardial Ischaemia and Reperfusion Injury in VIVO.Shock. 2016 Aug;46(2):202-13. doi: 10.1097/SHK.0000000000000578. Shock. 2016. PMID: 26844976
-
Ultramicronized palmitoylethanolamide in spinal cord injury neuropathic pain: a randomized, double-blind, placebo-controlled trial.Pain. 2016 Sep;157(9):2097-2103. doi: 10.1097/j.pain.0000000000000623. Pain. 2016. PMID: 27227691 Clinical Trial.
-
Mechanisms and clinical applications of palmitoylethanolamide (PEA) in the treatment of neuropathic pain.Inflammopharmacology. 2025 Jan;33(1):121-133. doi: 10.1007/s10787-024-01623-8. Epub 2024 Dec 23. Inflammopharmacology. 2025. PMID: 39714723 Review.
-
Role of palmitoylethanolamide (PEA) in depression: Translational evidence: Special Section on "Translational and Neuroscience Studies in Affective Disorders". Section Editor, Maria Nobile MD, PhD. This Section of JAD focuses on the relevance of translational and neuroscience studies in providing a better understanding of the neural basis of affective disorders. The main aim is to briefly summaries relevant research findings in clinical neuroscience with particular regards to specific innovative topics in mood and anxiety disorders.J Affect Disord. 2019 Aug 1;255:S0165-0327(18)31599-4. doi: 10.1016/j.jad.2018.10.117. Epub 2018 Oct 25. J Affect Disord. 2019. PMID: 30391203 Review.
Cited by
-
A Decades-Long Journey of Palmitoylethanolamide (PEA) for Chronic Neuropathic Pain Management: A Comprehensive Narrative Review.Pain Ther. 2025 Feb;14(1):81-101. doi: 10.1007/s40122-024-00685-4. Epub 2024 Dec 4. Pain Ther. 2025. PMID: 39630391 Free PMC article. Review.
-
Perineural Invasion in Pancreatic Ductal Adenocarcinoma: From Molecules towards Drugs of Clinical Relevance.Cancers (Basel). 2022 Nov 24;14(23):5793. doi: 10.3390/cancers14235793. Cancers (Basel). 2022. PMID: 36497277 Free PMC article. Review.
-
Treatment of Established Chemotherapy-Induced Neuropathy with N-Palmitoylethanolamide: A Randomized, Double-Blind Phase II Pilot Study.Cancers (Basel). 2024 Dec 20;16(24):4244. doi: 10.3390/cancers16244244. Cancers (Basel). 2024. PMID: 39766143 Free PMC article.
-
MCC950 Reduces the Anxiodepressive-like Behaviors and Memory Deficits Related to Paclitaxel-Induced Peripheral Neuropathy in Mice.Antioxidants (Basel). 2025 Jan 25;14(2):143. doi: 10.3390/antiox14020143. Antioxidants (Basel). 2025. PMID: 40002330 Free PMC article.
-
Role of peroxisome proliferator-activated receptor alpha in neurodegenerative diseases and other neurological disorders: Clinical application prospects.Neural Regen Res. 2026 Apr 1;21(4):1468-1482. doi: 10.4103/NRR.NRR-D-24-01371. Epub 2025 Jun 19. Neural Regen Res. 2026. PMID: 40537027 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

