Palmitic Acid Impedes Extravillous Trophoblast Activity by Increasing MRP1 Expression and Function
- PMID: 36009056
- PMCID: PMC9406058
- DOI: 10.3390/biom12081162
Palmitic Acid Impedes Extravillous Trophoblast Activity by Increasing MRP1 Expression and Function
Retraction in
-
RETRACTED: Ashar et al. Palmitic Acid Impedes Extravillous Trophoblast Activity by Increasing MRP1 Expression and Function. Biomolecules 2022, 12, 1162.Biomolecules. 2024 Apr 8;14(4):453. doi: 10.3390/biom14040453. Biomolecules. 2024. PMID: 38672526 Free PMC article.
Abstract
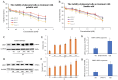
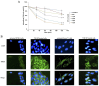
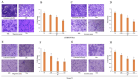
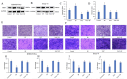
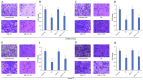
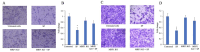
Normal function of placental extravillous trophoblasts (EVTs), which are responsible for uteroplacental vascular remodeling, is critical for adequate delivery of oxygen and nutrients to the developing fetus and normal fetal programming. Proliferation and invasion of spiral arteries by EVTs depends upon adequate levels of folate. Multidrug resistance-associated protein 1 (MRP1), which is an efflux transporter, is known to remove folate from these cells. We hypothesized that palmitic acid increases MRP1-mediated folate removal from EVTs, thereby interfering with EVTs' role in early placental vascular remodeling. HTR-8/SVneo and Swan-71 cells, first trimester human EVTs, were grown in the absence or presence of 0.5 mM and 0.7 mM palmitic acid, respectively, for 72 h. Palmitic acid increased ABCC1 gene expression and MRP1 protein expression in both cell lines. The rate of folate efflux from the cells into the media increased with a decrease in migration and invasion functions in the cultured cells. Treatment with N-acetylcysteine (NAC) prevented the palmitic acid-mediated upregulation of MRP1 and restored invasion and migration in the EVTs. Finally, in an ABCC1 knockout subline of Swan-71 cells, there was a significant increase in invasion and migration functions. The novel finding in this study that palmitic acid increases MRP1-mediated folate efflux provides a missing link that helps to explain how maternal consumption of saturated fatty acids compromises the in utero environment.
Keywords: MRP1; extravillous trophoblast; in utero environment; placenta; saturated fatty acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Palmitic acid induces inflammation in placental trophoblasts and impairs their migration toward smooth muscle cells through plasminogen activator inhibitor-1.Mol Hum Reprod. 2020 Nov 1;26(11):850-865. doi: 10.1093/molehr/gaaa061. Mol Hum Reprod. 2020. PMID: 32898274 Free PMC article.
-
Oleic acid stimulation of motility of human extravillous trophoblast cells is mediated by stearoyl-CoA desaturase-1 activity.Mol Hum Reprod. 2017 Nov 1;23(11):755-770. doi: 10.1093/molehr/gax051. Mol Hum Reprod. 2017. PMID: 29117333
-
LncRNA MEG3 inhibits trophoblast invasion and trophoblast-mediated VSMC loss in uterine spiral artery remodeling.Mol Reprod Dev. 2019 Jun;86(6):686-695. doi: 10.1002/mrd.23147. Epub 2019 May 8. Mol Reprod Dev. 2019. PMID: 31066488
-
Failure of physiological transformation and spiral artery atherosis: their roles in preeclampsia.Am J Obstet Gynecol. 2022 Feb;226(2S):S895-S906. doi: 10.1016/j.ajog.2020.09.026. Epub 2020 Sep 21. Am J Obstet Gynecol. 2022. PMID: 32971013 Review.
-
Function and control of human invasive trophoblast subtypes: Intrinsic vs. maternal control.Cell Adh Migr. 2016 Mar 3;10(1-2):154-62. doi: 10.1080/19336918.2015.1089376. Epub 2015 Sep 29. Cell Adh Migr. 2016. PMID: 26418186 Free PMC article. Review.
Cited by
-
RETRACTED: Ashar et al. Palmitic Acid Impedes Extravillous Trophoblast Activity by Increasing MRP1 Expression and Function. Biomolecules 2022, 12, 1162.Biomolecules. 2024 Apr 8;14(4):453. doi: 10.3390/biom14040453. Biomolecules. 2024. PMID: 38672526 Free PMC article.
-
FXR, MRP-1 and SLC7A5: New Targets for the Treatment of Hepatocellular Carcinoma.Technol Cancer Res Treat. 2024 Jan-Dec;23:15330338241276889. doi: 10.1177/15330338241276889. Technol Cancer Res Treat. 2024. PMID: 39194338 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

