Solitonic Windkessel Model for Intracranial Aneurysm
- PMID: 36009080
- PMCID: PMC9405599
- DOI: 10.3390/brainsci12081016
Solitonic Windkessel Model for Intracranial Aneurysm
Abstract
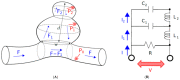
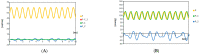
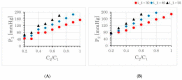
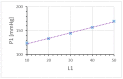
The Windkessel model, which is known as a successful model for explaining the hemodynamic circulation, is a mathematical model with a direct correspondence with the electric circuit. We propose a theoretical model for the intracranial aneurysm based on the Windkessel-type steady blood flow. Intracranial aneurysms are well known vascular lesions, which cause subarachnoid hemorrhages. Since an aneurysm is an end-sack formed on the blood vessel, it functions as an unusual blood path that has characteristic features such as a reservoir and bottle neck orifice. We simulate an aneurysm by an electric circuit consisting of three different impedances, resistance, capacitance and inductance. A dumbbell-shaped aneurysm is the most dangerous aneurysm to easily rupture. Our aneurysmal model is created as a two-story aneurysm model for this point, thus namely the five-element Windkessel. Then, the mathematical formula was solved in numerical simulations by changing the size of the aneurysm and the elasticity of the aneurysm wall. An analysis of this model provided that the presence of the daughter aneurysm and the thinning of the aneurysm wall are positively correlated with a sharp increase in blood pressure in the aneurysm dome. Our mathematic aneurysm model proposes a good analogue to the real aneurysm and proved that this model includes soliton that is a non-decreasing wave propagation.
Keywords: Windkessel model; intracranial aneurysm; soliton; subarachnoid hemorrhage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Ventriculomegaly without elevated intracranial pressure? Normal pressure hydrocephalus as a disorder of the cerebral windkessel.Front Neurol. 2025 May 1;16:1591275. doi: 10.3389/fneur.2025.1591275. eCollection 2025. Front Neurol. 2025. PMID: 40376155 Free PMC article.
-
Mathematical model of the rupture mechanism of intracranial saccular aneurysms through daughter aneurysm formation and growth.Neurol Res. 2005 Jul;27(5):459-65. doi: 10.1179/016164105X25171. Neurol Res. 2005. PMID: 15978170
-
Coil embolization for intracranial aneurysms: an evidence-based analysis.Ont Health Technol Assess Ser. 2006;6(1):1-114. Epub 2006 Jan 1. Ont Health Technol Assess Ser. 2006. PMID: 23074479 Free PMC article.
-
Diagnosis and Treatment of Unruptured Intracranial Aneurysms and Aneurysmal Subarachnoid Hemorrhage.Mayo Clin Proc. 2021 Jul;96(7):1970-2000. doi: 10.1016/j.mayocp.2021.01.005. Epub 2021 May 13. Mayo Clin Proc. 2021. PMID: 33992453 Review.
-
Preventive screening for intracranial aneurysms.Int J Stroke. 2022 Jan;17(1):30-36. doi: 10.1177/17474930211024584. Epub 2021 Jun 17. Int J Stroke. 2022. PMID: 34042530 Free PMC article. Review.
Cited by
-
Aneurysm Formation at the Internal Carotid Artery Bifurcation Is Related to the Vascular Geometry of the Bifurcation.Brain Sci. 2024 Dec 12;14(12):1247. doi: 10.3390/brainsci14121247. Brain Sci. 2024. PMID: 39766446 Free PMC article.
References
-
- Bender B.T., Wendt H., Monarch T., Beaty N., Lin L.M., Huang J., Coon A., Tamargo R.J., Colby G.P. Small aneurysms account for the majority and increasing percentage of aneurysmal subarachnoid hemorrhage: A 25-years, single institution study. Neurosurgery. 2018;83:692–699. doi: 10.1093/neuros/nyx484. - DOI - PubMed
-
- Ujiie H., Tachibana H., Hiramatsu O., Hazel A.L., Matsumoto T., Ogasawara Y., Nakajima H., Hori T., Takakura K., Kajiya F. Effect of size and shape (aspect ratio) on the hemodynamics of saccular aneurysms: A possible index for surgical treatment of intracranial aneurysms. Neurosurgery. 1999;45:119–130. - PubMed
LinkOut - more resources
Full Text Sources

