Use of Riluzole for the Treatment of Hereditary Ataxias: A Systematic Review
- PMID: 36009103
- PMCID: PMC9405857
- DOI: 10.3390/brainsci12081040
Use of Riluzole for the Treatment of Hereditary Ataxias: A Systematic Review
Abstract
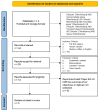
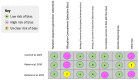
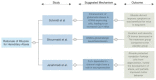
Ataxia is a constellation of symptoms that involves a lack of coordination, imbalance, and difficulty walking. Hereditary ataxia occurs when a person is born with defective genes, and this degenerative disorder may progress for several years. There is no effective cure for ataxia, so we need to search for new treatments. Recently, interest in riluzole in the treatment of ataxia has emerged. We conducted this systematic review to analyze the safety and efficacy of riluzole for treating hereditary ataxia in recent clinical trials. We conducted a systematic review using PubMed and Google Scholar as databases in search of this relationship. We used the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and Meta-analysis of Observational Studies in Epidemiology (MOOSE) protocols to conduct this study. For inclusion criteria, we included full-text clinical trials on humans written in English and found three clinical trials. We excluded case reports, literature reviews, systematic reviews, and meta-analyses for this analysis. We aimed to evaluate the Scale for the Assessment and Rating of Ataxia (SARA) score, the International Cooperative Ataxia Rating Scale (ICARS) score, and the safety of the medication. Two out of the three clinical trials showed statistically significant clinical improvement in the ICARS and SARA scores, while the other trial did not show improvement in the clinical or radiological outcomes. The drug was safe in all clinical trials. Overall, the results of this analysis of riluzole for the treatment of hereditary ataxia are encouraging. Further clinical trials are needed to investigate the efficacy of riluzole on hereditary ataxia.
Keywords: NMDA receptor antagonist; riluzole; spinocerebellar ataxia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources