The Effect of Noise Trauma and Deep Brain Stimulation of the Medial Geniculate Body on Tissue Activity in the Auditory Pathway
- PMID: 36009162
- PMCID: PMC9405782
- DOI: 10.3390/brainsci12081099
The Effect of Noise Trauma and Deep Brain Stimulation of the Medial Geniculate Body on Tissue Activity in the Auditory Pathway
Abstract
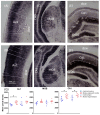
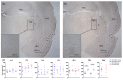
Tinnitus is defined as the phantom perception of sound. To date, there is no curative treatment, and contemporary treatments have failed to show beneficial outcomes. Deep brain stimulation has been suggested as a potential therapy for refractory tinnitus. However, the optimal target and stimulation regimens remain to be defined. Herein, we investigated metabolic and neuronal activity changes using cytochrome C oxidase histochemistry and c-Fos immunohistochemistry in a noise trauma-induced rat model of tinnitus. We also assessed changes in neuronal activity following medial geniculate body (MGB) high-frequency stimulation (HFS). Metabolic activity was reduced in the primary auditory cortex, MGB and CA1 region of the hippocampus in noise-exposed rats. Additionally, c-Fos expression was increased in the primary auditory cortex of those animals. Furthermore, MGB-HFS enhanced c-Fos expression in the thalamic reticular nucleus. We concluded that noise trauma alters tissue activity in multiple brain areas including the auditory and limbic regions. MGB-HFS resulted in higher neuronal activity in the thalamic reticular nucleus. Given the prominent role of the auditory thalamus in tinnitus, these data provide more rationales towards targeting the MGB with HFS as a symptom management tool in tinnitus.
Keywords: deep brain stimulation; medial geniculate body; tinnitus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Noise-induced neurophysiological alterations in the rat medial geniculate body and thalamocortical desynchronization by deep brain stimulation.J Neurophysiol. 2021 Feb 1;125(2):661-671. doi: 10.1152/jn.00752.2019. Epub 2021 Jan 6. J Neurophysiol. 2021. PMID: 33405997
-
Alleviation of Tinnitus With High-Frequency Stimulation of the Dorsal Cochlear Nucleus: A Rodent Study.Trends Hear. 2019 Jan-Dec;23:2331216519835080. doi: 10.1177/2331216519835080. Trends Hear. 2019. PMID: 30868944 Free PMC article.
-
Auditory thalamic circuits and GABAA receptor function: Putative mechanisms in tinnitus pathology.Hear Res. 2017 Jun;349:197-207. doi: 10.1016/j.heares.2016.08.009. Epub 2016 Aug 21. Hear Res. 2017. PMID: 27553899 Free PMC article. Review.
-
Sensory gating functions of the auditory thalamus: Adaptation and modulations through noise-exposure and high-frequency stimulation in rats.Behav Brain Res. 2023 Jul 26;450:114498. doi: 10.1016/j.bbr.2023.114498. Epub 2023 May 16. Behav Brain Res. 2023. PMID: 37201892
-
Auditory cortex stimulation to suppress tinnitus: mechanisms and strategies.Hear Res. 2013 Jan;295:38-57. doi: 10.1016/j.heares.2012.05.007. Epub 2012 Jun 6. Hear Res. 2013. PMID: 22683861 Review.
Cited by
-
Neural Plasticity in Tinnitus Mechanisms.Brain Sci. 2023 Nov 22;13(12):1615. doi: 10.3390/brainsci13121615. Brain Sci. 2023. PMID: 38137063 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

