Unraveling the Effects of Carotenoids Accumulation in Human Papillary Thyroid Carcinoma
- PMID: 36009182
- PMCID: PMC9405418
- DOI: 10.3390/antiox11081463
Unraveling the Effects of Carotenoids Accumulation in Human Papillary Thyroid Carcinoma
Abstract
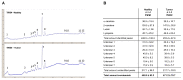
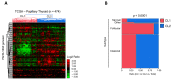
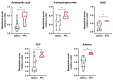
Among the thyroid cancers, papillary thyroid cancer (PTC) accounts for 90% of the cases. In addition to the necessity to identify new targets for PTC treatment, early diagnosis and management are highly demanded. Previous data indicated that the multivariate statistical analysis of the Raman spectra allows the discrimination of healthy tissues from PTC ones; this is characterized by bands typical of carotenoids. Here, we dissected the molecular effects of carotenoid accumulation in PTC patients by analyzing whether they were required to provide increased retinoic acid (RA) synthesis and signaling and/or to sustain antioxidant functions. HPLC analysis revealed the lack of a significant difference in the overall content of carotenoids. For this reason, we wondered whether the carotenoid accumulation in PTC patients could be related to vitamin A derivative retinoic acid (RA) biosynthesis and, consequently, the RA-related pathway activation. The transcriptomic analysis performed using a dedicated PCR array revealed a significant downregulation of RA-related pathways in PTCs, suggesting that the carotenoid accumulation in PTC could be related to a lower metabolic conversion into RA compared to that of healthy tissues. In addition, the gene expression profile of 474 PTC cases previously published in the framework of the Cancer Genome Atlas (TGCA) project was examined by hierarchical clustering and heatmap analyses. This metanalysis study indicated that the RA-related pathways resulted in being significantly downregulated in PTCs and being associated with the follicular variant of PTC (FV-PTC). To assess whether the possible fate of the carotenoids accumulated in PTCs is associated with the oxidative stress response, the expression of enzymes involved in ROS scavenging was checked. An increased oxidative stress status and a reduced antioxidant defense response were observed in PTCs compared to matched healthy thyroids; this was possibly associated with the prooxidant effects of high levels of carotenoids. Finally, the DepMap datasets were used to profile the levels of 225 metabolites in 12 thyroid cancer cell lines. The results obtained suggested that the high carotenoid content in PTCs correlates with tryptophan metabolism. This pilot provided novel possible markers and possible therapeutic targets for PTC diagnosis and therapy. For the future, a larger study including a higher number of PTC patients will be necessary to further validate the molecular data reported here.
Keywords: antioxidant; carotenoid; papillary thyroid cancer; retinoic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

