The Potential Role of m6A in the Regulation of TBI-Induced BGA Dysfunction
- PMID: 36009239
- PMCID: PMC9405408
- DOI: 10.3390/antiox11081521
The Potential Role of m6A in the Regulation of TBI-Induced BGA Dysfunction
Abstract
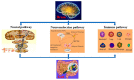
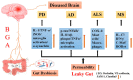
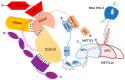
The brain-gut axis (BGA) is an important bidirectional communication pathway for the development, progress and interaction of many diseases between the brain and gut, but the mechanisms remain unclear, especially the post-transcriptional regulation of BGA after traumatic brain injury (TBI). RNA methylation is one of the most important modifications in post-transcriptional regulation. N6-methyladenosine (m6A), as the most abundant post-transcriptional modification of mRNA in eukaryotes, has recently been identified and characterized in both the brain and gut. The purpose of this review is to describe the pathophysiological changes in BGA after TBI, and then investigate the post-transcriptional bidirectional regulation mechanisms of TBI-induced BGA dysfunction. Here, we mainly focus on the characteristics of m6A RNA methylation in the post-TBI BGA, highlight the possible regulatory mechanisms of m6A modification in TBI-induced BGA dysfunction, and finally discuss the outcome of considering m6A as a therapeutic target to improve the recovery of the brain and gut dysfunction caused by TBI.
Keywords: brain-gut axis; m6A RNA modification; traumatic brain injury.
Conflict of interest statement
The authors declare that they have no conflict of interest. There are no competing interest of any nature to report. This research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Figures





References
-
- Singaravelu Jaganathan K., Sullivan K.A. Traumatic Brain Injury Rehabilitation: An Exercise Immunology Perspective. Exerc. Immunol. Rev. 2022;28:90–97. - PubMed
Publication types
Grants and funding
- 82172820, 82070541, 81771332, 81571184/National Natural Science Foundation of China
- 22ZR1451200/Natural Science Foundation of Shanghai
- PWR12018-07/the Outstanding Leaders Training Program of Pudong Health Bureau of Shanghai
- PWYgy 2021-07/the Medical Discipline Construction Project of Pudong Health Committee of Shanghai
- PWZxk 2017-23/the Key Discipline Construction Project of Pudong Health Bureau of Shanghai
LinkOut - more resources
Full Text Sources

