DJ-1 and SOD1 Act Independently in the Protection against Anoxia in Drosophila melanogaster
- PMID: 36009245
- PMCID: PMC9405364
- DOI: 10.3390/antiox11081527
DJ-1 and SOD1 Act Independently in the Protection against Anoxia in Drosophila melanogaster
Abstract
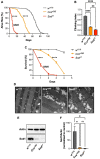
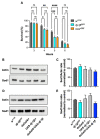
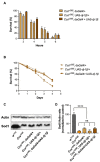
Redox homeostasis is a vital process the maintenance of which is assured by the presence of numerous antioxidant small molecules and enzymes and the alteration of which is involved in many pathologies, including several neurodegenerative disorders. Among the different enzymes involved in the antioxidant response, SOD1 and DJ-1 have both been associated with the pathogenesis of amyotrophic lateral sclerosis and Parkinson's disease, suggesting a possible interplay in their mechanism of action. Copper deficiency in the SOD1-active site has been proposed as a central determinant in SOD1-related neurodegeneration. SOD1 maturation mainly relies on the presence of the protein copper chaperone for SOD1 (CCS), but a CCS-independent alternative pathway also exists and functions under anaerobic conditions. To explore the possible involvement of DJ-1 in such a pathway in vivo, we exposed Drosophila melanogaster to anoxia and evaluated the effect of DJ-1 on fly survival and SOD1 levels, in the presence or absence of CCS. Loss of DJ-1 negatively affects the fly response to the anoxic treatment, but our data indicate that the protective activity of DJ-1 is independent of SOD1 in Drosophila, indicating that the two proteins may act in different pathways.
Keywords: DJ-1; Drosophila melanogaster; Parkinson’s disease; SOD1; amyotrophic lateral sclerosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Roberts B.R., Lim N.K.H., McAllum E.J., Donnelly P.S., Hare D.J., Doble P.A., Turner B.J., Price K.A., Lim S.C., Paterson B.M., et al. Oral Treatment with Cu (II) (Atsm) Increases Mutant SOD1 in Vivo but Protects Motor Neurons and Improves the Phenotype of a Transgenic Mouse Model of Amyotrophic Lateral Sclerosis. J. Neurosci. 2014;34:8021–8031. doi: 10.1523/JNEUROSCI.4196-13.2014. - DOI - PMC - PubMed
-
- Williams J.R., Trias E., Beilby P.R., Lopez N.I., Labut E.M., Bradford C.S., Roberts B.R., McAllum E.J., Crouch P.J., Rhoads T.W., et al. Copper Delivery to the CNS by Cu ATSM Effectively Treats Motor Neuron Disease in SOD (G93A) Mice Co-Expressing the Copper-Chaperone-for-SOD. Neurobiol. Dis. 2016;89:1–9. doi: 10.1016/j.nbd.2016.01.020. - DOI - PMC - PubMed
-
- Hilton J.B., Mercer S.W., Lim N.K.H., Faux N.G., Buncic G., Beckman J.S., Roberts B.R., Donnelly P.S., White A.R., Crouch P.J. Cu II (Atsm) Improves the Neurological Phenotype and Survival of SOD1 G93A Mice and Selectively Increases Enzymatically Active SOD1 in the Spinal Cord. Sci. Rep. 2017;7:42292. doi: 10.1038/srep42292. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

