Vitamin C Deficiency in Blood Samples of COVID-19 Patients
- PMID: 36009299
- PMCID: PMC9405075
- DOI: 10.3390/antiox11081580
Vitamin C Deficiency in Blood Samples of COVID-19 Patients
Abstract
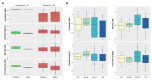
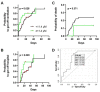
Coronavirus disease 2019 (COVID-19) is the most notable pandemic of the modern era. A relationship between ascorbate (vitamin C) and COVID-19 severity is well known, whereas the role of other vitamins is less understood. The present study compared the blood levels of four vitamins in a cohort of COVID-19 patients with different severities and uninfected individuals. Serum concentrations of ascorbate, calcidiol, retinol, and α-tocopherol were measured in a cohort of 74 COVID-19 patients and 8 uninfected volunteers. The blood levels were statistically compared and additional co-morbidity factors were considered. COVID-19 patients had significantly lower plasma ascorbate levels than the controls (p-value < 0.001), and further stratification revealed that the controls had higher levels than fatal, critical, and severe COVID-19 cases (p-values < 0.001). However, no such trend was observed for calcidiol, retinol, or α-tocopherol (p-value ≥ 0.093). Survival analysis showed that plasma ascorbate below 11.4 µM was associated with a lengthy hospitalization and a high risk of death. The results indicated that COVID-19 cases had depleted blood ascorbate associated with poor medical conditions, confirming the role of this vitamin in the outcome of COVID-19 infection.
Keywords: COVID-19; ascorbate; calcidiol; retinol; vitamin plasma levels; α-tocopherol.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Johns Hopkins University Coronavirus Research Center, Center for Systems Science and Engineering (CSSE) Baltimore, MD, USA. [(accessed on 1 April 2022)]. Available online: https://coronavirus.jhu.edu/map.html.
Grants and funding
LinkOut - more resources
Full Text Sources

