Oxidative Implications of Substituting a Conserved Cysteine Residue in Sugar Beet Phytoglobin BvPgb 1.2
- PMID: 36009334
- PMCID: PMC9404779
- DOI: 10.3390/antiox11081615
Oxidative Implications of Substituting a Conserved Cysteine Residue in Sugar Beet Phytoglobin BvPgb 1.2
Abstract
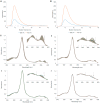
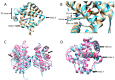
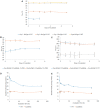
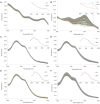
Phytoglobins (Pgbs) are plant-originating heme proteins of the globin superfamily with varying degrees of hexacoordination. Pgbs have a conserved cysteine residue, the role of which is poorly understood. In this paper, we investigated the functional and structural role of cysteine in BvPgb1.2, a Class 1 Pgb from sugar beet (Beta vulgaris), by constructing an alanine-substituted mutant (Cys86Ala). The substitution had little impact on structure, dimerization, and heme loss as determined by X-ray crystallography, size-exclusion chromatography, and an apomyoglobin-based heme-loss assay, respectively. The substitution significantly affected other important biochemical properties. The autoxidation rate increased 16.7- and 14.4-fold for the mutant versus the native protein at 25 °C and 37 °C, respectively. Thermal stability similarly increased for the mutant by ~2.5 °C as measured by nano-differential scanning fluorimetry. Monitoring peroxidase activity over 7 days showed a 60% activity decrease in the native protein, from 33.7 to 20.2 U/mg protein. When comparing the two proteins, the mutant displayed a remarkable enzymatic stability as activity remained relatively constant throughout, albeit at a lower level, ~12 U/mg protein. This suggests that cysteine plays an important role in BvPgb1.2 function and stability, despite having seemingly little effect on its tertiary and quaternary structure.
Keywords: autoxidation; crystallization; heme loss; hexacoordination; peroxidase activity; phytoglobin; thermal stability.
Conflict of interest statement
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Olson J., Mathews A.J., Rohlfs R.J., Springer B.A., Egeberg K.D., Sligar S.G., Tame J., Renaud J.-P., Nagai K. The role of the distal histidine in myoglobin and haemoglobin. Nature. 1988;336:265–266. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

