Antioxidant Therapy Significantly Attenuates Hepatotoxicity following Low Dose Exposure to Microcystin-LR in a Murine Model of Diet-Induced Non-Alcoholic Fatty Liver Disease
- PMID: 36009344
- PMCID: PMC9404967
- DOI: 10.3390/antiox11081625
Antioxidant Therapy Significantly Attenuates Hepatotoxicity following Low Dose Exposure to Microcystin-LR in a Murine Model of Diet-Induced Non-Alcoholic Fatty Liver Disease
Abstract
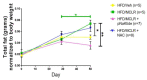
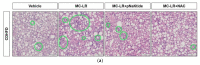
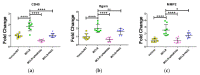
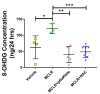
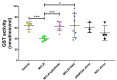
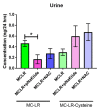
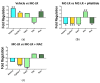
We have previously shown in a murine model of Non-alcoholic Fatty Liver Disease (NAFLD) that chronic, low-dose exposure to the Harmful Algal Bloom cyanotoxin microcystin-LR (MC-LR), resulted in significant hepatotoxicity including micro-vesicular lipid accumulation, impaired toxin metabolism as well as dysregulation of the key signaling pathways involved in inflammation, immune response and oxidative stress. On this background we hypothesized that augmentation of hepatic drug metabolism pathways with targeted antioxidant therapies would improve MC-LR metabolism and reduce hepatic injury in NAFLD mice exposed to MC-LR. We chose N-acetylcysteine (NAC, 40 mM), a known antioxidant that augments the glutathione detoxification pathway and a novel peptide (pNaKtide, 25 mg/kg) which is targeted to interrupting a specific Src-kinase mediated pro-oxidant amplification mechanism. Histological analysis showed significant increase in hepatic inflammation in NAFLD mice exposed to MC-LR which was attenuated on treatment with both NAC and pNaKtide (both p ≤ 0.05). Oxidative stress, as measured by 8-OHDG levels in urine and protein carbonylation in liver sections, was also significantly downregulated upon treatment with both antioxidants after MC-LR exposure. Genetic analysis of key drug transporters including Abcb1a, Phase I enzyme-Cyp3a11 and Phase II metabolic enzymes-Pkm (Pyruvate kinase, muscle), Pklr (Pyruvate kinase, liver, and red blood cell) and Gad1 (Glutamic acid decarboxylase) was significantly altered by MC-LR exposure as compared to the non-exposed control group (all p ≤ 0.05). These changes were significantly attenuated with both pNaKtide and NAC treatment. These results suggest that MC-LR metabolism and detoxification is significantly impaired in the setting of NAFLD, and that these pathways can potentially be reversed with targeted antioxidant treatment.
Keywords: microcystin-LR (MC-LR); n-acetylcysteine (NAC); non-alcoholic fatty liver disease (NAFLD); pNaKtide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Büdel B. Plant Desiccation Tolerance. Springer; Berlin/Heidelberg, Germany: 2011. Cyanobacteria: Habitats and species; pp. 11–21.
-
- Mantzouki E., Lürling M., Fastner J., de Senerpont Domis L., Wilk-Woźniak E., Koreivienė J., Seelen L., Teurlincx S., Verstijnen Y., Krztoń W., et al. Temperature effects explain continental scale distribution of cyanobacterial toxins. Toxins. 2018;10:156. doi: 10.3390/toxins10040156. - DOI - PMC - PubMed
-
- Ueno Y., Nagat S., Suttajit M., Mebs D., Vasconcelos V. Immunochemical survey of microcystins in environmental water in various countries. Mycotoxins Phycotoxins—Dev. Chem. Toxicol. Food Saf. P. 1998:449–453.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

