Melatonin Decreases Acute Inflammatory Response to Neural Probe Insertion
- PMID: 36009346
- PMCID: PMC9405074
- DOI: 10.3390/antiox11081628
Melatonin Decreases Acute Inflammatory Response to Neural Probe Insertion
Abstract
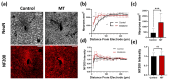
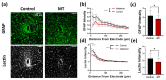
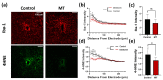
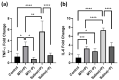
Neural electrode insertion trauma impedes the recording and stimulation capabilities of numerous diagnostic and treatment avenues. Implantation leads to the activation of inflammatory markers and cell types, which is detrimental to neural tissue health and recording capabilities. Oxidative stress and inflammation at the implant site have been shown to decrease with chronic administration of antioxidant melatonin at week 16, but its effects on the acute landscape have not been studied. To assess the effect of melatonin administration in the acute phase, specifically the first week post-implantation, we utilized histological and q-PCR methods to quantify cellular and molecular indicators of inflammation and oxidative stress in the tissue surrounding implanted probes in C57BL/6 mice as well as two-photon microscopy to track the microglial responses to the probes in real-time in transgenic mice expressing GFP with CX3CR1 promotor. Histological results indicate that melatonin effectively maintained neuron density surrounding the electrode, inhibited accumulation and activation of microglia and astrocytes, and reduced oxidative tissue damage. The expression of the pro-inflammatory cytokines, TNF-α and IL-6, were significantly reduced in melatonin-treated animals. Additionally, microglial encapsulation of the implant surface was inhibited by melatonin as compared to control animals following implantation. Our results combined with previous research suggest that melatonin is a particularly suitable drug for modulating inflammatory activity around neural electrode implants both acutely and chronically, translating to more stable and reliable interfaces.
Keywords: anti-inflammatory; antioxidant; glial scar; insertion trauma; melatonin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Melatonin improves quality and longevity of chronic neural recording.Biomaterials. 2018 Oct;180:225-239. doi: 10.1016/j.biomaterials.2018.07.026. Epub 2018 Jul 17. Biomaterials. 2018. PMID: 30053658 Free PMC article.
-
Dexamethasone retrodialysis attenuates microglial response to implanted probes in vivo.Biomaterials. 2016 May;87:157-169. doi: 10.1016/j.biomaterials.2016.02.013. Epub 2016 Feb 10. Biomaterials. 2016. PMID: 26923363 Free PMC article.
-
Neuroadhesive L1 coating attenuates acute microglial attachment to neural electrodes as revealed by live two-photon microscopy.Biomaterials. 2017 Jan;113:279-292. doi: 10.1016/j.biomaterials.2016.10.054. Epub 2016 Nov 1. Biomaterials. 2017. PMID: 27837661 Free PMC article.
-
Comprehensive characterization and failure modes of tungsten microwire arrays in chronic neural implants.J Neural Eng. 2012 Oct;9(5):056015. doi: 10.1088/1741-2560/9/5/056015. Epub 2012 Sep 25. J Neural Eng. 2012. PMID: 23010756
-
Oxidative and inflammatory biomarkers of ischemia and reperfusion injuries.Dan Med J. 2015 Apr;62(4):B5054. Dan Med J. 2015. PMID: 25872540 Review.
Cited by
-
Aberrant accumulation of age- and disease-associated factors following neural probe implantation in a mouse model of Alzheimer's disease.J Neural Eng. 2023 Sep 1;20(4):046044. doi: 10.1088/1741-2552/aceca5. J Neural Eng. 2023. PMID: 37531953 Free PMC article.
-
Stable in-vivo electrochemical sensing of tonic serotonin levels using PEDOT/CNT-coated glassy carbon flexible microelectrode arrays.Biosens Bioelectron. 2023 Jun 15;230:115242. doi: 10.1016/j.bios.2023.115242. Epub 2023 Mar 21. Biosens Bioelectron. 2023. PMID: 36989659 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

