Effect of N-3 Polyunsaturated Fatty Acids on Lipid Composition in Familial Hypercholesterolemia: A Randomized Crossover Trial
- PMID: 36009356
- PMCID: PMC9405021
- DOI: 10.3390/biomedicines10081809
Effect of N-3 Polyunsaturated Fatty Acids on Lipid Composition in Familial Hypercholesterolemia: A Randomized Crossover Trial
Abstract
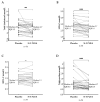
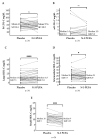
Individuals with familial hypercholesterolemia (FH) have an increased risk of cardiovascular disease. Treatment is mainly low-density lipoprotein cholesterol (LDL-C) reduction. How omega-3 polyunsaturated fatty acids (n-3 PUFAs) supplements affect lipoproteins in FH subjects is unknown. We hypothesized that a high-dose n-3 PUFA supplement would reduce atherogenic lipoproteins and influence the high-density lipoprotein cholesterol (HDL-C) function. We performed a randomized, double-blinded crossover study with 34 genetically verified FH individuals (18−75 years, clinically stable, statin treatment > 12 months). Treatment was 4 g n-3 PUFAs (1840 mg eicosapentaenoic acid and 1520 mg docosahexaenoic acid daily) or four capsules of olive oil for three months in a crossover design with a washout period of three months. The defined outcomes were changes in triglycerides, lipoproteins, lipoprotein subfractions, apolipoproteins, and HDL-C function. After treatment with n-3 PUFAs, total cholesterol, LDL-C, and triglycerides were reduced compared to placebo (p ≤ 0.01 for all). Total HDL-C levels were unchanged, but the subfraction of large HDL-C was higher (p ≤ 0.0001) after n-3 PUFAs than after placebo, and intermediate HDL-C and small HDL-C were reduced after n-3 PUFAs compared to placebo (p = 0.02 and p ≤ 0.001, respectively). No changes were found in apolipoproteins and HDL-C function. N-3 PUFAs supplements reduced atherogenic lipoproteins in FH subjects, leaving HDL-C function unaffected.
Keywords: cardiovascular disease; familial hypercholesterolemia; lipids; omega-3 fatty acids; randomized trial.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

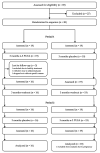
References
-
- Duell P.B., Gidding S.S., Andersen R.L., Knickelbine T., Anderson L., Gianos E., Shrader P., Kindt I., O’Brien E.C., McCann D., et al. Longitudinal low density lipoprotein cholesterol goal achievement and cardiovascular outcomes among adult patients with familial hypercholesterolemia: The CASCADE FH registry. Atherosclerosis. 2019;289:85–93. doi: 10.1016/j.atherosclerosis.2019.08.007. - DOI - PubMed
-
- Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L., Chapman M.J., De Backer G.G., Delgado V., Ference B.A., et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. - DOI - PubMed
-
- Yokoyama M., Origasa H., Matsuzaki M., Matsuzawa Y., Saito Y., Ishikawa Y., Oikawa S., Sasaki J., Hishida H., Itakura H., et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): A randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. - DOI - PubMed
-
- Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet. 1999;354:447–455. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

