Increased Stiffness Downregulates Focal Adhesion Kinase Expression in Pancreatic Cancer Cells Cultured in 3D Self-Assembling Peptide Scaffolds
- PMID: 36009384
- PMCID: PMC9405295
- DOI: 10.3390/biomedicines10081835
Increased Stiffness Downregulates Focal Adhesion Kinase Expression in Pancreatic Cancer Cells Cultured in 3D Self-Assembling Peptide Scaffolds
Abstract
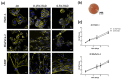
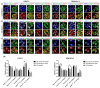
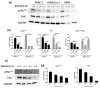
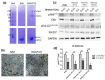
The focal adhesion kinase (FAK) is a non-receptor tyrosine kinase that participates in integrin-mediated signal transduction and contributes to different biological processes, such as cell migration, survival, proliferation and angiogenesis. Moreover, FAK can be activated by autophosphorylation at position Y397 and trigger different signaling pathways in response to increased extracellular matrix stiffness. In addition, FAK is overexpressed and/or hyperactivated in many epithelial cancers, and its expression correlates with tumor malignancy and invasion potential. One of the characteristics of solid tumors is an over deposition of ECM components, which generates a stiff microenvironment that promotes, among other features, sustained cell proliferation and survival. Researchers are, therefore, increasingly developing cell culture models to mimic the increased stiffness associated with these kinds of tumors. In the present work, we have developed a new 3D in vitro model to study the effect of matrix stiffness in pancreatic ductal adenocarcinoma (PDAC) cells as this kind of tumor is characterized by a desmoplastic stroma and an increased stiffness compared to its normal counterpart. For that, we have used a synthetic self-assembling peptide nanofiber matrix, RAD16-I, which does not suffer a significant degradation in vitro, thus allowing to maintain the same local stiffness along culture time. We show that increased matrix stiffness in synthetic 3D RAD16-I gels, but not in collagen type I scaffolds, promotes FAK downregulation at a protein level in all the cell lines analyzed. Moreover, even though it has classically been described that stiff 3D matrices promote an increase in pFAKY397/FAK proteins, we found that this ratio in soft and stiff RAD16-I gels is cell-type-dependent. This study highlights how cell response to increased matrix stiffness greatly depends on the nature of the matrix used for 3D culture.
Keywords: FAK; PDAC; RAD16-I; biomechanics; pancreatic ductal adenocarcinoma; self-assembling peptides; stiffness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The extracellular matrix and focal adhesion kinase signaling regulate cancer stem cell function in pancreatic ductal adenocarcinoma.PLoS One. 2017 Jul 10;12(7):e0180181. doi: 10.1371/journal.pone.0180181. eCollection 2017. PLoS One. 2017. PMID: 28692661 Free PMC article.
-
Erlotinib Promotes Ligand-Induced EGFR Degradation in 3D but Not 2D Cultures of Pancreatic Ductal Adenocarcinoma Cells.Cancers (Basel). 2021 Sep 7;13(18):4504. doi: 10.3390/cancers13184504. Cancers (Basel). 2021. PMID: 34572731 Free PMC article.
-
Stiff collagen matrices increase tumorigenic prolactin signaling in breast cancer cells.J Biol Chem. 2013 May 3;288(18):12722-32. doi: 10.1074/jbc.M112.447631. Epub 2013 Mar 24. J Biol Chem. 2013. PMID: 23530035 Free PMC article.
-
Focal adhesion kinase and its potential involvement in tumor invasion and metastasis.Head Neck. 1998 Dec;20(8):745-52. doi: 10.1002/(sici)1097-0347(199812)20:8<745::aid-hed14>3.0.co;2-z. Head Neck. 1998. PMID: 9790298 Review.
-
Role of focal adhesion kinase in integrin signaling.Int J Biochem Cell Biol. 1997 Aug-Sep;29(8-9):1085-96. doi: 10.1016/s1357-2725(97)00051-4. Int J Biochem Cell Biol. 1997. PMID: 9416004 Review.
Cited by
-
Shape-Morphing Photoresponsive Hydrogels Reveal Dynamic Topographical Conditioning of Fibroblasts.Adv Sci (Weinh). 2023 Nov;10(31):e2303136. doi: 10.1002/advs.202303136. Epub 2023 Sep 23. Adv Sci (Weinh). 2023. PMID: 37740666 Free PMC article.
-
Tumour microenvironment in pheochromocytoma and paraganglioma.Front Endocrinol (Lausanne). 2023 Mar 22;14:1137456. doi: 10.3389/fendo.2023.1137456. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37033265 Free PMC article. Review.
-
Exploring the mechanism of fibronectin extra domain B in the tumor microenvironment and implications for targeted immunotherapy and diagnostics (Review).Mol Med Rep. 2025 Jun;31(6):160. doi: 10.3892/mmr.2025.13525. Epub 2025 Apr 11. Mol Med Rep. 2025. PMID: 40211711 Free PMC article. Review.
-
The influence of biophysical niche on tumor-associated macrophages in liver cancer.Hepatol Commun. 2024 Oct 30;8(11):e0569. doi: 10.1097/HC9.0000000000000569. eCollection 2024 Nov 1. Hepatol Commun. 2024. PMID: 39470328 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

