High Levels of Progesterone Receptor B in MCF-7 Cells Enable Radical Anti-Tumoral and Anti-Estrogenic Effect of Progestin
- PMID: 36009407
- PMCID: PMC9405688
- DOI: 10.3390/biomedicines10081860
High Levels of Progesterone Receptor B in MCF-7 Cells Enable Radical Anti-Tumoral and Anti-Estrogenic Effect of Progestin
Abstract
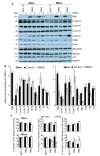
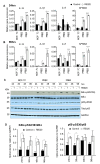
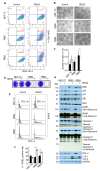
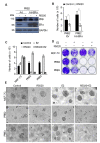
The widely reported conflicting effects of progestin on breast cancer suggest that the progesterone receptor (PR) has dual functions depending on the cellular context. Cell models that enable PR to fully express anti-tumoral properties are valuable for the understanding of molecular determinant(s) of the anti-tumoral property. This study evaluated whether the expression of high levels of PR in MCF-7 cells enabled a strong anti-tumoral response to progestin. MCF-7 cells were engineered to overexpress PRB by stable transfection. A single dose of Promegestone (R5020) induced an irreversible cell growth arrest and senescence-associated secretory phenotype in MCF-7 cells with PRB overexpression (MCF-7PRB cells) but had no effect on MCF-7 cells with PRA overexpression. The growth-arresting effect was associated with downregulations of cyclin A2 and B1, CDK2, and CDK4 despite an initial upregulation of cyclin A2 and B1. R5020 also induced an evident activation of Nuclear Factor κB (NF-κB) and upregulation of interleukins IL-1α, IL-1β, and IL-8. Although R5020 caused a significant increase of CD24+CD44+ cell population, R5020-treated MCF-7PRB cells were unable to form tumorspheres and underwent massive apoptosis, which is paradoxically associated with marked downregulations of the pro-apoptotic proteins BID, BAX, PARP, and Caspases 7 and 8, as well as diminution of anti-apoptotic protein BCL-2. Importantly, R5020-activated PRB abolished the effect of estrogen. This intense anti-estrogenic effect was mediated by marked downregulation of ERα and pioneer factor FOXA1, leading to diminished chromatin-associated ERα and FOXA1 and estrogen-induced target gene expression. In conclusion, high levels of agonist-activated PRB in breast cancer cells can be strongly anti-tumoral and anti-estrogenic despite the initial unproductive cell cycle acceleration. Repression of ERα and FOXA1 expression is a major mechanism for the strong anti-estrogenic effect.
Keywords: MCF-7; antiestrogenic; breast cancer; progesterone receptor; progestin; replicative senescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Rossouw J.E., Anderson G.L., Prentice R.L., LaCroix A.Z., Kooperberg C., Stefanick M.L., Jackson R.D., Beresford S.A., Howard B.V., Johnson K.C., et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

