Internal Ribosome Entry Site (IRES)-Mediated Translation and Its Potential for Novel mRNA-Based Therapy Development
- PMID: 36009412
- PMCID: PMC9405587
- DOI: 10.3390/biomedicines10081865
Internal Ribosome Entry Site (IRES)-Mediated Translation and Its Potential for Novel mRNA-Based Therapy Development
Abstract
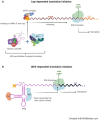
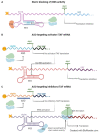
Many conditions can benefit from RNA-based therapies, namely, those targeting internal ribosome entry sites (IRESs) and their regulatory proteins, the IRES trans-acting factors (ITAFs). IRES-mediated translation is an alternative mechanism of translation initiation, known for maintaining protein synthesis when canonical translation is impaired. During a stress response, it contributes to cell reprogramming and adaptation to the new environment. The relationship between IRESs and ITAFs with tumorigenesis and resistance to therapy has been studied in recent years, proposing new therapeutic targets and treatments. In addition, IRES-dependent translation initiation dysregulation is also related to neurological and cardiovascular diseases, muscular atrophies, or other syndromes. The participation of these structures in the development of such pathologies has been studied, yet to a far lesser extent than in cancer. Strategies involving the disruption of IRES-ITAF interactions or the modification of ITAF expression levels may be used with great impact in the development of new therapeutics. In this review, we aim to comprehend the current data on groups of human pathologies associated with IRES and/or ITAF dysregulation and their application in the designing of new therapeutic approaches using them as targets or tools. Thus, we wish to summarise the evidence in the field hoping to open new promising lines of investigation toward personalised treatments.
Keywords: IRES trans-acting factors; IRES-based multicistronic vectors; RNA-based therapies; antisense oligonucleotides; internal ribosome entry sites.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
hnRNP K Is a Novel Internal Ribosomal Entry Site-Transacting Factor That Negatively Regulates Foot-and-Mouth Disease Virus Translation and Replication and Is Antagonized by Viral 3C Protease.J Virol. 2020 Aug 17;94(17):e00803-20. doi: 10.1128/JVI.00803-20. Print 2020 Aug 17. J Virol. 2020. PMID: 32581104 Free PMC article.
-
IRES Trans-Acting Factors, Key Actors of the Stress Response.Int J Mol Sci. 2019 Feb 20;20(4):924. doi: 10.3390/ijms20040924. Int J Mol Sci. 2019. PMID: 30791615 Free PMC article. Review.
-
Hepatitis C Virus Translation Regulation.Int J Mol Sci. 2020 Mar 27;21(7):2328. doi: 10.3390/ijms21072328. Int J Mol Sci. 2020. PMID: 32230899 Free PMC article. Review.
-
Control of the negative IRES trans-acting factor KHSRP by ubiquitination.Nucleic Acids Res. 2017 Jan 9;45(1):271-287. doi: 10.1093/nar/gkw1042. Epub 2016 Nov 28. Nucleic Acids Res. 2017. PMID: 27899653 Free PMC article.
-
Regulation Mechanisms of Viral IRES-Driven Translation.Trends Microbiol. 2017 Jul;25(7):546-561. doi: 10.1016/j.tim.2017.01.010. Epub 2017 Feb 24. Trends Microbiol. 2017. PMID: 28242053 Review.
Cited by
-
Non-canonical translation in cancer: significance and therapeutic potential of non-canonical ORFs, m6A-modification, and circular RNAs.Cell Death Discov. 2024 Sep 27;10(1):412. doi: 10.1038/s41420-024-02185-y. Cell Death Discov. 2024. PMID: 39333489 Free PMC article. Review.
-
A novel function of the M2 muscarinic receptor.Trends Pharmacol Sci. 2024 Aug;45(8):663-665. doi: 10.1016/j.tips.2024.05.010. Epub 2024 Jun 8. Trends Pharmacol Sci. 2024. PMID: 38853101 Free PMC article.
-
Molecular mechanisms of circular RNA translation.Exp Mol Med. 2024 Jun;56(6):1272-1280. doi: 10.1038/s12276-024-01220-3. Epub 2024 Jun 14. Exp Mol Med. 2024. PMID: 38871818 Free PMC article. Review.
-
Transcriptional profiling of human cartilage endplate cells identifies novel genes and cell clusters underlying degenerated and non-degenerated phenotypes.Arthritis Res Ther. 2024 Jan 3;26(1):12. doi: 10.1186/s13075-023-03220-6. Arthritis Res Ther. 2024. PMID: 38173036 Free PMC article.
-
Enabling mRNA Therapeutics: Current Landscape and Challenges in Manufacturing.Biomolecules. 2023 Oct 9;13(10):1497. doi: 10.3390/biom13101497. Biomolecules. 2023. PMID: 37892179 Free PMC article. Review.
References
-
- Hua Y., Krainer A.R. Spinal Muscular Atrophy Disease Mechanisms and Therapy. Academic Press; Cambridge, MA, USA: 2017. Antisense-Oligonucleotide Modulation of SMN2 Pre-mRNA Splicing; pp. 301–311. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

