Stabilization of F-Actin Cytoskeleton by Paclitaxel Improves the Blastocyst Developmental Competence through P38 MAPK Activity in Porcine Embryos
- PMID: 36009414
- PMCID: PMC9405004
- DOI: 10.3390/biomedicines10081867
Stabilization of F-Actin Cytoskeleton by Paclitaxel Improves the Blastocyst Developmental Competence through P38 MAPK Activity in Porcine Embryos
Abstract
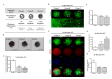
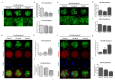
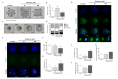
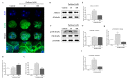
Changes in F-actin distribution and cortical F-actin morphology are important for blastocyst developmental competence during embryogenesis. However, the effect of paclitaxel as a microtubule stabilizer on embryonic development in pigs remains unclear. We investigated the role of F-actin cytoskeleton stabilization via P38 MAPK activation using paclitaxel to improve the developmental potential of blastocysts in pigs. In this study, F-actin enrichment and adducin expression based on blastomere fragment rate and cytokinesis defects were investigated in cleaved embryos after in vitro fertilization (IVF). Adducin and adhesive junction F-actin fluorescence intensity were significantly reduced with increasing blastomere fragment rate in porcine embryos. In addition, porcine embryos were cultured with 10 and 100 nM paclitaxel for two days after IVF. Adhesive junction F-actin stabilization and p-P38 MAPK activity in embryos exposed to 10 nM paclitaxel increased significantly with blastocyst development competence. However, increased F-actin aggregation, cytokinesis defects, and over-expression of p-P38 MAPK protein by 100 nM paclitaxel exposure disrupted blastocyst development in porcine embryos. In addition, exposure to 100 nM paclitaxel increased the misaligned α-tubulin of spindle assembly and adhesive junction F-actin aggregation at the blastocyst stage, which might be caused by p-P38 protein over-expression-derived apoptosis in porcine embryos.
Keywords: F-actin; P38 MAPK; adducin; cytoskeleton; paclitaxel; porcine embryo.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Morphologic evaluation and actin filament distribution in porcine embryos produced in vitro and in vivo.Biol Reprod. 1999 Apr;60(4):1020-8. doi: 10.1095/biolreprod60.4.1020. Biol Reprod. 1999. PMID: 10084980
-
GDF8 activates p38 MAPK signaling during porcine oocyte maturation in vitro.Theriogenology. 2017 Oct 1;101:123-134. doi: 10.1016/j.theriogenology.2017.06.003. Epub 2017 Jun 6. Theriogenology. 2017. PMID: 28708509
-
Lineage segregation in human pre-implantation embryos is specified by YAP1 and TEAD1.Hum Reprod. 2023 Aug 1;38(8):1484-1498. doi: 10.1093/humrep/dead107. Hum Reprod. 2023. PMID: 37295962
-
Rapamycin encourages the maintenance of mitochondrial dynamic balance and mitophagy activity for improving developmental competence of blastocysts in porcine embryos in vitro.Mol Reprod Dev. 2023 Apr;90(4):236-247. doi: 10.1002/mrd.23681. Epub 2023 Mar 21. Mol Reprod Dev. 2023. PMID: 36944102
-
A pre-in vitro maturation medium containing cumulus oocyte complex ligand-receptor signaling molecules maintains meiotic arrest, supports the cumulus oocyte complex and improves oocyte developmental competence.Mol Hum Reprod. 2017 Sep 1;23(9):594-606. doi: 10.1093/molehr/gax032. Mol Hum Reprod. 2017. PMID: 28586460
Cited by
-
Effect of the Rho-Kinase/ROCK Signaling Pathway on Cytoskeleton Components.Genes (Basel). 2023 Jan 20;14(2):272. doi: 10.3390/genes14020272. Genes (Basel). 2023. PMID: 36833199 Free PMC article. Review.
-
Nicotinamide mononucleotide biosynthesis and the F-actin cytoskeleton regulate spindle assembly and oocyte maturation quality in post-ovulatory aged porcine oocytes.Cell Commun Signal. 2025 Apr 17;23(1):186. doi: 10.1186/s12964-025-02200-4. Cell Commun Signal. 2025. PMID: 40247324 Free PMC article.
References
-
- Buey R.M., Barasoain I., Jackson E., Meyer A., Giannakakou P., Paterson I., Mooberry S., Andreu J.M., Díaz J.F. Microtubule Interactions with Chemically Diverse Stabilizing Agents: Thermodynamics of Binding to The Paclitaxel Site Predicts Cytotoxicity. Chem. Biol. 2005;12:1269–1279. doi: 10.1016/j.chembiol.2005.09.010. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

