Soluble (Pro)Renin Receptor Levels Are Regulated by Plasma Renin Activity and Correlated with Edema in Mice and Humans with HFrEF
- PMID: 36009420
- PMCID: PMC9405551
- DOI: 10.3390/biomedicines10081874
Soluble (Pro)Renin Receptor Levels Are Regulated by Plasma Renin Activity and Correlated with Edema in Mice and Humans with HFrEF
Abstract
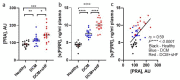
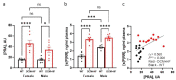
Symptomatic heart failure with reduced ejection fraction (HFrEF) is characterized by edema and chronic pathological activation of the classical renin-angiotensin-aldosterone system (RAAS). The soluble (pro)renin receptor (s(P)RR) is released into circulation by proteolytic cleavage of tissue expressed (P)RR and is a candidate biomarker of RAAS activation. However, previous studies linked elevated levels of s(P)RR in patients with HFrEF to renal dysfunction. Utilizing prospectively enrolled patients with comparable rEF, we show that increased plasma levels of s(P)RR are associated with symptomatic HF (characterized by edema), independent of chronic renal dysfunction. We also found that s(P)RR levels were positively correlated with patient plasma renin activity (PRA). Normotensive mice with dilated cardiomyopathy (DCM) and HFrEF, without renal dysfunction, showed plasma s(P)RR and PRA patterns similar to human HFrEF patients. Plasma s(P)RR levels positively correlated with PRA and systemic edema, but not with EF, resembling findings in patients with HFrEF without chronic kidney dysfunction. In female DCM mice with elevated PRA levels and plasma s(P)RR levels, a randomized, blinded trial comparing the direct renin inhibitor, aliskiren vs. vehicle control, showed that direct renin inhibition normalized PRA, lowered s(P)RR, and prevented symptomatic HFrEF. Considered in light of previous findings, these data suggest that, in HFrEF, in the absence of renal dysfunction, elevation of plasma s(P)RR levels is caused by increased PRA and associated with the development of systemic edema.
Keywords: HFrEF; edema; renin plasma activity; soluble (pro)renin receptor.
Conflict of interest statement
A patent application has been filed on the basis of this study, as indicated above.
Figures



Similar articles
-
Elevated plasma soluble (pro)renin receptor levels are associated with left ventricular remodeling and renal function in chronic heart failure patients with reduced ejection fraction.Peptides. 2019 Jan;111:152-157. doi: 10.1016/j.peptides.2018.04.010. Epub 2018 Apr 13. Peptides. 2019. PMID: 29660382
-
Renin Activity in Heart Failure with Reduced Systolic Function-New Insights.Int J Mol Sci. 2019 Jun 28;20(13):3182. doi: 10.3390/ijms20133182. Int J Mol Sci. 2019. PMID: 31261774 Free PMC article. Review.
-
Plasma renin activity in patients with heart failure and reduced ejection fraction on optimal medical therapy.J Renin Angiotensin Aldosterone Syst. 2017 Jul-Sep;18(3):1470320317729919. doi: 10.1177/1470320317729919. J Renin Angiotensin Aldosterone Syst. 2017. PMID: 28875746 Free PMC article.
-
Normalizing Plasma Renin Activity in Experimental Dilated Cardiomyopathy: Effects on Edema, Cachexia, and Survival.Int J Mol Sci. 2019 Aug 9;20(16):3886. doi: 10.3390/ijms20163886. Int J Mol Sci. 2019. PMID: 31404946 Free PMC article.
-
Renin-Angiotensin System Inhibition, Worsening Renal Function, and Outcome in Heart Failure Patients With Reduced and Preserved Ejection Fraction: A Meta-Analysis of Published Study Data.Circ Heart Fail. 2017 Feb;10(2):e003588. doi: 10.1161/CIRCHEARTFAILURE.116.003588. Circ Heart Fail. 2017. PMID: 28209765 Review.
Cited by
-
OXGR1-Dependent (Pro)Renin Receptor Upregulation in Collecting Ducts of the Clipped Kidney Contributes to Na+ Balance in Goldblatt Hypertensive Mice.Int J Mol Sci. 2024 Sep 18;25(18):10045. doi: 10.3390/ijms251810045. Int J Mol Sci. 2024. PMID: 39337535 Free PMC article.
-
Falling corin and ANP activity levels accelerate development of heart failure and cardiac fibrosis.Front Cardiovasc Med. 2023 Apr 18;10:1120487. doi: 10.3389/fcvm.2023.1120487. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 37388639 Free PMC article. No abstract available.
References
-
- Vaduganathan M., Claggett B.L., Jhund P.S., Cunningham J.W., Pedro Ferreira J., Zannad F., Packer M., Fonarow G.C., McMurray J.J.V., Solomon S.D. Estimating Lifetime Benefits of Comprehensive Disease-Modifying Pharmacological Therapies in Patients with Heart Failure with Reduced Ejection Fraction: A Comparative Analysis of Three Randomised Controlled Trials. Lancet. 2020;396:121–128. doi: 10.1016/S0140-6736(20)30748-0. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

