Sex-Based Evaluation of Lipid Profile in Postoperative Adjuvant Mitotane Treatment for Adrenocortical Carcinoma
- PMID: 36009421
- PMCID: PMC9405852
- DOI: 10.3390/biomedicines10081873
Sex-Based Evaluation of Lipid Profile in Postoperative Adjuvant Mitotane Treatment for Adrenocortical Carcinoma
Abstract
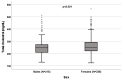
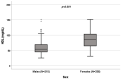
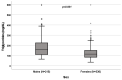
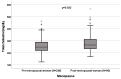
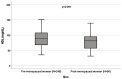
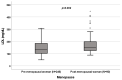
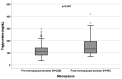
Background: A wide interindividual variability in mitotane concentrations and treatment-related dyslipidemia have been reported. Here, we aimed to underline the sex-related differences in the lipid profile in patients that underwent radical surgery of adrenocortical carcinoma during treatment with adjuvant mitotane. Methods: A chromatographic method was used to quantify the drug in plasma collected from adult patients with complete tumor resection, also considering active metabolite o,p’-DDE. Results: We observed different lipid profiles between males and females and between pre- and post-menopausal women. Considering the mitotane-related effects on lipid levels, we observed that higher drug concentrations were correlated with higher HDL in all the considered groups (p < 0.001), with total cholesterol both in males (p = 0.005) and females (p = 0.036), with triglycerides in postmenopausal females (p = 0.002) and with LDL in male patients (p < 0.001). Increases in o,p’-DDE were positively correlated with HDL levels in all the groups (p < 0.001) and negatively with LDL in all the groups (males p = 0.008, pre- and post-menopausal females p < 0.001), with total cholesterol in pre- (p = 0.016) and post-menopausal women (p = 0.01) and with triglycerides in premenopausal females (p = 0.005). Conclusions: This is the first study designed to evaluate sex differences in lipoprotein and lipid levels during mitotane adjuvant treatment; the results suggest that a gender and personalized approach could be useful to prevent and manage alterations in the lipid profile.
Keywords: cholesterol; dyslipidemia; gender; menopause; o,p’-DDD; o,p’-DDE; safety; sex; side effect; triglycerides.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Sex Differences on Mitotane Concentration and Treatment Outcome in Patients with Adrenocortical Carcinoma.Life (Basel). 2021 Mar 23;11(3):266. doi: 10.3390/life11030266. Life (Basel). 2021. PMID: 33807024 Free PMC article.
-
Circannual variation of mitotane and its metabolites plasma levels in patients with adrenocortical carcinoma.J Pharm Pharmacol. 2017 Nov;69(11):1524-1530. doi: 10.1111/jphp.12798. Epub 2017 Aug 15. J Pharm Pharmacol. 2017. PMID: 28809444
-
Association of mitotane with chylomicrons and serum lipoproteins: practical implications for treatment of adrenocortical carcinoma.Eur J Endocrinol. 2016 Mar;174(3):343-53. doi: 10.1530/EJE-15-0946. Epub 2015 Dec 15. Eur J Endocrinol. 2016. PMID: 26671975
-
[Metabolism of o,p'-DDD (mitotane) in human and animals. Actual notions and practical deductions (author's transl)].Ann Endocrinol (Paris). 1977;38(1):13-25. Ann Endocrinol (Paris). 1977. PMID: 324349 Review. French.
-
Metabolic and Endocrine Toxicities of Mitotane: A Systematic Review.Cancers (Basel). 2021 Oct 5;13(19):5001. doi: 10.3390/cancers13195001. Cancers (Basel). 2021. PMID: 34638485 Free PMC article. Review.
References
-
- Fassnacht M., Dekkers O.M., Else T., Baudin E., Berruti A., de Krijger R., Haak H.R., Mihai R., Assie G., Terzolo M. European Society of Endocrinology Clinical Practice Guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2018;179:G1–G46. doi: 10.1530/EJE-18-0608. - DOI - PubMed
-
- Fassnacht M., Assie G., Baudin E., Eisenhofer G., de la Fouchardiere C., Haak H.R., de Krijger R., Porpiglia F., Terzolo M., Berruti A., et al. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020;31:1476–1490. doi: 10.1016/j.annonc.2020.08.2099. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

