Multiple Sclerosis: Enzymatic Cross Site-Specific Recognition and Hydrolysis of H2A Histone by IgGs against H2A, H1, H2B, H3 Histones, Myelin Basic Protein, and DNA
- PMID: 36009424
- PMCID: PMC9405453
- DOI: 10.3390/biomedicines10081876
Multiple Sclerosis: Enzymatic Cross Site-Specific Recognition and Hydrolysis of H2A Histone by IgGs against H2A, H1, H2B, H3 Histones, Myelin Basic Protein, and DNA
Abstract
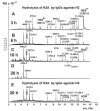
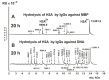
Histones have a paramount role in chromatin remodeling and gene transcription. Free histones are damage-associated molecules in the blood; administration of histones to animals drives systemic inflammatory and toxic effects. Myelin basic protein (MBP) is the most crucial component of the axon myelin-proteolipid sheath. Antibodies-abzymes with different enzymatic activities are very toxic and an essential feature of some autoimmune diseases. Electrophoretically homogeneous IgGs against H1, H2A, H2B, H3, H4, MBP, and DNA were derived from sera of multiple sclerosis (MS) patients by several affinity chromatographies. Using MALDI-TOFF mass spectrometry, it was shown that IgGs against H2A split H2A at 12 sites; the number of H2A hydrolysis sites by antibodies against other antigens is different: H1 (19), H2B (11), H3 (15), H4 (9), MBP (10), and DNA (23), and they only partly match. Thus, the complex formation polyreactivity and the enzymatic cross-activity of pernicious humans IgGs against five histones, MBP, and DNA have been shown for the first time. The data obtained indicate that the formation of such polyspecific-polyreactive abzymes, whose single active center can recognize and hydrolyze different substrates, can occur due to the formation of antibodies against hybrid antigenic determinants consisting of several histone protein sequences. IgGs with high affinity for DNA with DNase and protease activities may be antibodies against DNA-histone complex antigenic determinants, including protein and DNA sequences. Polyreactive IgGs-abzymes against MBP, five histones, and DNA with extended cytotoxicity can play a very negative role in the pathogenesis of multiple sclerosis and probably other different diseases.
Keywords: DNA; H1; H2A; H3; H4 histones; IgGs against H2B; catalytic abzymes; enzymatic cross recognition and hydrolysis; human blood sera antibodies-abzymes; hydrolysis of H2A histone; multiple sclerosis patients; myelin basic protein.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Multiple Sclerosis: Enzymatic Cross Site-Specific Recognition and Hydrolysis of H3 Histone by IgGs against H3, H1, H2A, H2B, H4 Histones, Myelin Basic Protein, and DNA.Biomedicines. 2022 Oct 21;10(10):2663. doi: 10.3390/biomedicines10102663. Biomedicines. 2022. PMID: 36289924 Free PMC article.
-
Multiple Sclerosis: Enzymatic Cross Site-Specific Hydrolysis of H1 Histone by IgGs against H1, H2A, H2B, H3, H4 Histones, and Myelin Basic Protein.Biomolecules. 2021 Aug 2;11(8):1140. doi: 10.3390/biom11081140. Biomolecules. 2021. PMID: 34439806 Free PMC article.
-
EAE of Mice: Enzymatic Cross Site-Specific Hydrolysis of H2A Histone by IgGs against H2A, H1, H2B, H3, and H4 Histones and Myelin Basic Protein.Int J Mol Sci. 2023 May 12;24(10):8636. doi: 10.3390/ijms24108636. Int J Mol Sci. 2023. PMID: 37239982 Free PMC article.
-
Immune Response and Production of Abzymes in Patients with Autoimmune and Neurodegenerative Diseases.Biochemistry (Mosc). 2025 Jan;90(Suppl 1):S373-S400. doi: 10.1134/S0006297924604167. Biochemistry (Mosc). 2025. PMID: 40164167 Review.
-
Primate-specific histone variants.Genome. 2021 Apr;64(4):337-346. doi: 10.1139/gen-2020-0094. Epub 2020 Nov 27. Genome. 2021. PMID: 33245240 Review.
Cited by
-
Exploring the theranostic potentials of miRNA and epigenetic networks in autoimmune diseases: A comprehensive review.Immun Inflamm Dis. 2023 Dec;11(12):e1121. doi: 10.1002/iid3.1121. Immun Inflamm Dis. 2023. PMID: 38156400 Free PMC article. Review.
-
Multiple Sclerosis: Enzymatic Cross Site-Specific Recognition and Hydrolysis of H3 Histone by IgGs against H3, H1, H2A, H2B, H4 Histones, Myelin Basic Protein, and DNA.Biomedicines. 2022 Oct 21;10(10):2663. doi: 10.3390/biomedicines10102663. Biomedicines. 2022. PMID: 36289924 Free PMC article.
-
Experimental Autoimmune Encephalomyelitis of Mice: IgGs from the Sera of Mice Hydrolyze miRNAs.Int J Mol Sci. 2023 Feb 23;24(5):4433. doi: 10.3390/ijms24054433. Int J Mol Sci. 2023. PMID: 36901861 Free PMC article.
References
-
- David B.S. In: Catalytic Antibodies. Keinan E., editor. Wiley-VCH Verlag GmbH and Co. KgaA; Weinheim, Germany: 2005. pp. 1–586.
-
- Nevinsky G.A., Buneva V.N. Natural catalytic antibodies–abzymes. In: Keinan E., editor. Catalytic Antibodies. VCH-Wiley Press; Weinheim, Germany: 2005. pp. 505–569.
-
- Nevinsky G.A. In: Natural catalytic antibodies in norm and in autoimmune diseases, In Autoimmune Diseases: Symptoms, Diagnosis and Treatment. Brenner K.J., editor. Nova Science Publishers Inc.; New York, NY, USA: 2010. pp. 1–107.
-
- Nevinsky G.A. Natural catalytic antibodies in norm and in HIV-infected patients. In: Kasenga F.H., editor. Understanding HIV/AIDS Management and Care—Pandemic Approaches the 21st Century. InTech; Rijeka, Croatia: 2011. pp. 151–192.
-
- Nevinsky G.A. Autoimmune processes in multiple sclerosis: Production of harmful catalytic antibodies associated with significant changes in the hematopoietic stem cell differentiation and proliferation. In: Conzalez-Quevedo A., editor. Multiple Sclerosis. InTech; Rijeka, Croatia: 2016. pp. 100–147.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

