Differential Effects of Anti-TNFα and Anti-α4β7 Drugs on Circulating Dendritic Cells Migratory Capacity in Inflammatory Bowel Disease
- PMID: 36009431
- PMCID: PMC9405461
- DOI: 10.3390/biomedicines10081885
Differential Effects of Anti-TNFα and Anti-α4β7 Drugs on Circulating Dendritic Cells Migratory Capacity in Inflammatory Bowel Disease
Abstract
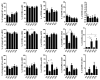
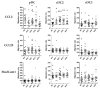
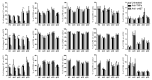
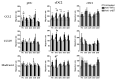
Inflammatory bowel disease (IBD) is an idiopathic and chronic disorder that includes ulcerative colitis (UC) and Crohn's disease (CD). Both diseases show an uncontrolled intestinal immune response that generates tissue inflammation. Dendritic cells (DCs) are antigen-presenting cells that play a key role in tolerance maintenance in the gastrointestinal mucosa. Although it has been reported that DC recruitment by the intestinal mucosa is more prominent in IBD patients, the specific mechanisms governing this migration are currently unknown. In this study, the expression of several homing markers and the migratory profile of circulating DC subsets towards intestinal chemo-attractants were evaluated and the effect of biological drugs with different mechanisms of action, such as anti-TNFα or anti-integrin α4β7 (vedolizumab), on this mechanism in healthy controls (HCs) and IBD patients was also assessed. Our results revealed that type 2 conventional DCs (cDC2) express differential homing marker profiles in UC and CD patients compared to HCs. Indeed, integrin β7 was differentially modulated by vedolizumab in CD and UC. Additionally, although CCL2 displayed a chemo-attractant effect over cDC2, while biological therapies did not modulate the expression of the homing markers, we paradoxically found that anti-TNF-treated cDC2 increased their migratory capacity towards CCL2 in HCs and IBD. Our results therefore suggest a key role for cDC2 migration towards the intestinal mucosa in IBD, something that could be explored in order to develop novel diagnostic biomarkers or to unravel new immunomodulatory targets in IBD.
Keywords: anti-TNFα; biological drugs; dendritic cells; inflammatory bowel disease; intestinal mucosa; migration; vedolizumab.
Conflict of interest statement
Gisbert has served as speaker, consultant and advisory member for/or has received research funding from MSD, Abbvie, Pfizer, Kern Pharma, Biogen, Mylan, Takeda, Janssen, Roche, Sandoz, Celgene/Bristol Myers, Gilead/Galapagos, Lilly, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, Norgine and Vifor Pharma. María Chaparro has served as a speaker and as consultant. She has received research and/or education funding from MSD, Abbvie, Hospira, Pfizer, Takeda, Janssen, Ferring, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Biogen, Gilead and Lilly.
Figures

References
-
- Chaparro M., Acosta M.B.-D., Benítez J.M., Cabriada J.L., Casanova M.J., Ceballos D., Esteve M., Fernández H., Ginard D., Gomollón F., et al. EpidemIBD: Rationale and Design of a Large-Scale Epidemiological Study of Inflammatory Bowel Disease in Spain. Ther. Adv. Gastroenterol. 2019;12:1756284819847034. doi: 10.1177/1756284819847034. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

