miRNome and Proteome Profiling of Small Extracellular Vesicles Secreted by Human Glioblastoma Cell Lines and Primary Cancer Stem Cells
- PMID: 36009432
- PMCID: PMC9405730
- DOI: 10.3390/biomedicines10081886
miRNome and Proteome Profiling of Small Extracellular Vesicles Secreted by Human Glioblastoma Cell Lines and Primary Cancer Stem Cells
Abstract
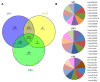
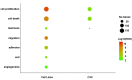
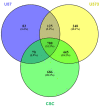
Glioblastoma (GBM) is the most common and aggressive brain tumor in adults. Despite available therapeutic interventions, it is very difficult to treat, and a cure is not yet available. The intra-tumoral GBM heterogeneity is a crucial factor contributing to poor clinical outcomes. GBM derives from a small heterogeneous population of cancer stem cells (CSCs). In cancer tissue, CSCs are concentrated within the so-called niches, where they progress from a slowly proliferating phase. CSCs, as most tumor cells, release extracellular vesicles (EVs) into the surrounding microenvironment. To explore the role of EVs in CSCs and GBM tumor cells, we investigated the miRNA and protein content of the small EVs (sEVs) secreted by two GBM-established cell lines and by GBM primary CSCs using omics analysis. Our data indicate that GBM-sEVs are selectively enriched for miRNAs that are known to display tumor suppressor activity, while their protein cargo is enriched for oncoproteins and tumor-associated proteins. Conversely, among the most up-regulated miRNAs in CSC-sEVs, we also found pro-tumor miRNAs and proteins related to stemness, cell proliferation, and apoptosis. Collectively, our findings support the hypothesis that sEVs selectively incorporate different miRNAs and proteins belonging both to fundamental processes (e.g., cell proliferation, cell death, stemness) as well as to more specialized ones (e.g., EMT, membrane docking, cell junction organization, ncRNA processing).
Keywords: cancer stem cells; extracellular vesicles; glioblastoma; miRNAs; proteome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Cenciarelli C., Marei H.E., Zonfrillo M., Casalbore P., Felsani A., Giannetti S., Trevisi G., Althani A., Mangiola A. The interference of Notch1 target Hes1 affects cell growth, differentiation, and invasiveness of Glioblastoma stem cells through modulation of oncogenic signals. Oncotarget. 2017;8:17873–17886. doi: 10.18632/oncotarget.15013. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

