Combination of Dexamethasone and Tofacitinib Reduces Xenogeneic MSC-Induced Immune Responses in a Mouse Model of Alzheimer's Disease
- PMID: 36009433
- PMCID: PMC9405531
- DOI: 10.3390/biomedicines10081882
Combination of Dexamethasone and Tofacitinib Reduces Xenogeneic MSC-Induced Immune Responses in a Mouse Model of Alzheimer's Disease
Abstract
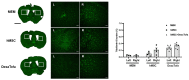
We have recently reported on how transplantation of human mesenchymal stem cells (MSCs) into the mouse parenchyma generated immune responses. To facilitate the clinical translation of MSC-based AD therapy, the safety and efficacy of human derived MSCs (hMSCs) must be confirmed in the pre-clinical stage. Thus, it is imperative to investigate measures to reduce immune responses exerted via xenotransplantation. In this study, immunosuppressants were co-administered to mice that had received injections of hMSCs into the parenchyma. Prior to performing experiments using transgenic AD mice (5xFAD), varying immunosuppressant regimens were tested in wild-type (WT) mice and the combination of dexamethasone and tofacitinib (DexaTofa) revealed to be effective in enhancing the persistence of hMSCs. According to transcriptome sequencing and immunohistochemical analyses, administration of DexaTofa reduced immune responses generated via transplantation of hMSCs in the parenchyma of 5xFAD mice. Significant mitigation of amyloid burden, however, was not noted following transplantation of hMSCs alone or hMSCs with DexaTofa. The efficacy of the immunosuppressant regimen should be tested in multiple AD mouse models to promote its successful application and use in AD stem cell therapy.
Keywords: Alzheimer’s disease; dexamethasone; immune response; immunosuppressant; tofacitinib.
Conflict of interest statement
The authors declare that they have no potential conflict of interest. Both the funders and ENCell Co., Ltd. had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures







References
-
- Hwang J.W., Lee N.K., Yang J.H., Son H.J., Bang S.I., Chang J.W., Na D.L. A Comparison of Immune Responses Exerted Following Syngeneic, Allogeneic, and Xenogeneic Transplantation of Mesenchymal Stem Cells into the Mouse Brain. Int. J. Mol. Sci. 2020;21:3052. doi: 10.3390/ijms21093052. - DOI - PMC - PubMed
-
- Hwang J.W., Myeong S.H., Lee N.H., Kim H., Son H.J., Chang J.W., Lee N.K., Na D.L. Immunosuppressant Drugs Mitigate Immune Responses Generated by Human Mesenchymal Stem Cells Transplanted into the Mouse Parenchyma. Cell Transpl. 2021;30:9636897211019025. doi: 10.1177/09636897211019025. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

