In Vivo and Ex Vivo Gene Electrotransfer in Ophthalmological Disorders
- PMID: 36009435
- PMCID: PMC9405572
- DOI: 10.3390/biomedicines10081889
In Vivo and Ex Vivo Gene Electrotransfer in Ophthalmological Disorders
Abstract
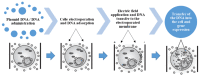
The aim of this document is to present an overview of gene electrotransfer in ophthalmological disorders. In order to ensure an adequate variety of the assessed studies, several electronic databases were considered and studies published between January 1998 and December 2021 were analysed. Three investigators carried out data extraction and analysis, focusing on both technical (i.e., electrical protocol, type of electrode, plasmid) and medical (i.e., type of study, threated disease) aspects and highlighting the main differences in terms of results obtained. Moreover, the IGEA experience in the project "Transposon-based, targeted ex vivo gene therapy to treat age-related macular degeneration" (TargetAMD) was reported in the results section. No clinical trial was found on international literature and on ClinicalTrials.gov. Twelve preclinical studies were found including in vivo and ex-vivo applications. The studied showed that electrotransfer could be very efficient for plasmid DNA transfection. Many attempts such as modification of the electric field, buffers and electrodes have been made and the optimization of electric field setting seems to be very important. Using this technique, gene replacement can be designed in cases of retinal inheritance or corneal disease and a wide range of human eye diseases could, in the future, benefitfrom these gene therapy technologies.
Keywords: ex-vivo; gene electrotransfer; in vivo; ophthalmological disorders.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

