The Hypoxia-Adenosine Link during Myocardial Ischemia-Reperfusion Injury
- PMID: 36009485
- PMCID: PMC9405579
- DOI: 10.3390/biomedicines10081939
The Hypoxia-Adenosine Link during Myocardial Ischemia-Reperfusion Injury
Abstract
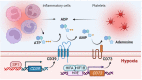
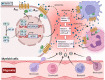
Despite increasing availability and more successful interventional approaches to restore coronary reperfusion, myocardial ischemia-reperfusion injury is a substantial cause of morbidity and mortality worldwide. During myocardial ischemia, the myocardium becomes profoundly hypoxic, thus causing stabilization of hypoxia-inducible transcription factors (HIF). Stabilization of HIF leads to a transcriptional program that promotes adaptation to hypoxia and cellular survival. Transcriptional consequences of HIF stabilization include increases in extracellular production and signaling effects of adenosine. Extracellular adenosine functions as a signaling molecule via the activation of adenosine receptors. Several studies implicated adenosine signaling in cardioprotection, particularly through the activation of the Adora2a and Adora2b receptors. Adenosine receptor activation can lead to metabolic adaptation to enhance ischemia tolerance or dampen myocardial reperfusion injury via signaling events on immune cells. Many studies highlight that clinical strategies to target the hypoxia-adenosine link could be considered for clinical trials. This could be achieved by using pharmacologic HIF activators or by directly enhancing extracellular adenosine production or signaling as a therapy for patients with acute myocardial infarction, or undergoing cardiac surgery.
Keywords: A2A; A2B; Adora2a; Adora2b; CD39; CD73; ENT1; ENT2; adenosine; hypoxia.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures



References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

