Short-Term Cortical Electrical Stimulation during the Acute Stage of Traumatic Brain Injury Improves Functional Recovery
- PMID: 36009512
- PMCID: PMC9405844
- DOI: 10.3390/biomedicines10081965
Short-Term Cortical Electrical Stimulation during the Acute Stage of Traumatic Brain Injury Improves Functional Recovery
Abstract
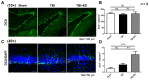
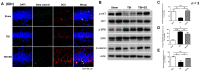
Functional restoration is an important issue in the treatment of traumatic brain injury (TBI). Various electrical stimulation devices and protocols have been applied in preclinical studies and have shown therapeutic potential for brain trauma. Short-term invasive cortical electrical stimulation during the acute stage of TBI might be a feasible adjuvant therapy for patients with moderate-to-severe brain injury receiving neurosurgical treatment in the intensive care unit. However, the therapeutic effects of short-term multisession cortical electrical stimulation for brain trauma are not clear. This study explored the therapeutic effects of acute-stage short-term cortical electrical stimulation on TBI. We conducted seven sessions of one-hour cortical electrical stimulation from day 0 to day 6 in rats after brain trauma by controlled cortical impact and then evaluated the functional outcome and histopathological changes. Our data showed that short-term cortical electrical stimulation improved motor coordination, short-term memory, and learning ability and attenuated neurological severity after brain trauma. Lesion volume, apoptosis, and gliosis after brain trauma were reduced, and trauma-induced neurogenesis in the hippocampus for the innate neural reparative response was increased. Our study demonstrated that short-term cortical electrical stimulation applied in the acute stage of traumatic brain injury is a potential adjuvant therapy to improve the recovery of neurological deficits.
Keywords: cortical electrical stimulation; functional recovery; neural stem cells; traumatic brain injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Treatment of traumatic brain injury in rats with N-acetyl-seryl-aspartyl-lysyl-proline.J Neurosurg. 2017 Mar;126(3):782-795. doi: 10.3171/2016.3.JNS152699. Epub 2016 May 20. J Neurosurg. 2017. PMID: 28245754 Free PMC article.
-
Cortical Electrical Stimulation Ameliorates Traumatic Brain Injury-Induced Sensorimotor and Cognitive Deficits in Rats.Front Neural Circuits. 2021 Jun 14;15:693073. doi: 10.3389/fncir.2021.693073. eCollection 2021. Front Neural Circuits. 2021. PMID: 34194304 Free PMC article.
-
Cortical Re-organization After Traumatic Brain Injury Elicited Using Functional Electrical Stimulation Therapy: A Case Report.Front Neurosci. 2021 Aug 19;15:693861. doi: 10.3389/fnins.2021.693861. eCollection 2021. Front Neurosci. 2021. PMID: 34489624 Free PMC article.
-
Neurostimulation for traumatic brain injury.J Neurosurg. 2014 Nov;121(5):1219-31. doi: 10.3171/2014.7.JNS131826. Epub 2014 Aug 29. J Neurosurg. 2014. PMID: 25170668 Review.
-
[Mild traumatic brain injury and postconcussive syndrome: a re-emergent questioning].Encephale. 2012 Sep;38(4):329-35. doi: 10.1016/j.encep.2011.07.003. Epub 2011 Aug 31. Encephale. 2012. PMID: 22980474 Review. French.
Cited by
-
Inflammation, brain connectivity, and neuromodulation in post-traumatic headache.Brain Behav Immun Health. 2024 Jan 6;35:100723. doi: 10.1016/j.bbih.2024.100723. eCollection 2024 Feb. Brain Behav Immun Health. 2024. PMID: 38292321 Free PMC article. Review.
-
Inhibition of Voltage-Gated Na+ Currents Exerted by KB-R7943 (2-[2-[4-(4-nitrobenzyloxy)phenyl]ethyl]isothiourea), an Inhibitor of Na+-Ca2+ Exchanging Process.Int J Mol Sci. 2023 Jan 16;24(2):1805. doi: 10.3390/ijms24021805. Int J Mol Sci. 2023. PMID: 36675319 Free PMC article.
-
Constructing organoid-brain-computer interfaces for neurofunctional repair after brain injury.Nat Commun. 2024 Nov 6;15(1):9580. doi: 10.1038/s41467-024-53858-2. Nat Commun. 2024. PMID: 39505863 Free PMC article.
-
Efficacy of adding selective electrical muscle stimulation to usual physical therapy for Bell's palsy: immediate and six-month outcomes.Eur J Transl Myol. 2023 Oct 24;33(4):11630. doi: 10.4081/ejtm.2023.11630. Eur J Transl Myol. 2023. PMID: 37877154 Free PMC article.
-
Transcriptomic analysis of rat brain response to alternating current electrical stimulation: unveiling insights via single-nucleus RNA sequencing.MedComm (2020). 2024 Mar 15;5(4):e514. doi: 10.1002/mco2.514. eCollection 2024 Apr. MedComm (2020). 2024. PMID: 38495123 Free PMC article.
References
-
- Compagnone C., d’Avella D., Servadei F., Angileri F.F., Brambilla G., Conti C., Cristofori L., Delfini R., Denaro L., Ducati A., et al. Patients with moderate head injury: A prospective multicenter study of 315 patients. Neurosurgery. 2009;64:690–696. doi: 10.1227/01.NEU.0000340796.18738.F7. discussion 696–697. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

