Silencing of LLGL2 Suppresses the Estradiol-Induced BPH-1 Cell Proliferation through the Regulation of Autophagy
- PMID: 36009528
- PMCID: PMC9406103
- DOI: 10.3390/biomedicines10081981
Silencing of LLGL2 Suppresses the Estradiol-Induced BPH-1 Cell Proliferation through the Regulation of Autophagy
Abstract
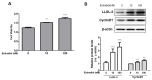
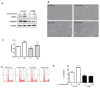
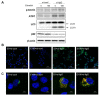
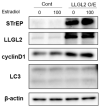
Lethal giant larvae (Lgl) is an apical-basal polarity gene first identified in Drosophila. LLGL2 is one of the mammalian homologs of Lgl. However, little is known about its function in the prostate. In this study, to explore the new role of LLGL2 in the prostate, we examined the proliferative activity of a BPH-1 cell line, a well-established model for the human prostate biology of benign prostatic hyperplasia (BPH). The expression of LLGL2 was dose-dependently increased in BPH-1 cells after treatment with 17β-estradiol (E2). Additionally, E2 treatment increased the proliferation of the BPH-1 cells. However, the knockdown of LLGL2 with siRNA significantly suppressed the proliferation of the E2-treated BPH-1 cells. Moreover, si-llgl2 treatment up-regulated the expression of LC-3B, ATG7, and p-beclin, which are known to play a pivotal role in autophagosome formation in E2-treated BPH-1 cells. Overexpression of LLGL2 was able to further prove these findings by showing the opposite results from the knockdown of LLGL2 in E2-treated BPH-1 cells. Collectively, our results suggest that LLGL2 is closely involved in the proliferation of prostate cells by regulating autophagosome formation. These results provide a better understanding of the mechanism involved in the effect of LLGL2 on prostate cell proliferation. LLGL2 might serve as a potential target in the diagnosis and/or treatment of human BPH.
Keywords: LLGL2; autophagosome formation; benign prostatic hyperplasia; proliferation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Novel role of LLGL2 silencing in autophagy: reversing epithelial-mesenchymal transition in prostate cancer.Biol Res. 2024 May 8;57(1):25. doi: 10.1186/s40659-024-00499-w. Biol Res. 2024. PMID: 38720397 Free PMC article.
-
Cynomorium songaricum Rupr demonstrates phytoestrogenic or phytoandrogenic like activities that attenuates benign prostatic hyperplasia via regulating steroid 5-α-reductase.J Ethnopharmacol. 2019 May 10;235:65-74. doi: 10.1016/j.jep.2019.01.038. Epub 2019 Jan 29. J Ethnopharmacol. 2019. PMID: 30708032
-
LLGL2 Increases Ca2+ Influx and Exerts Oncogenic Activities via PI3K/AKT Signaling Pathway in Hepatocellular Carcinoma.Front Oncol. 2021 Jun 10;11:683629. doi: 10.3389/fonc.2021.683629. eCollection 2021. Front Oncol. 2021. PMID: 34178676 Free PMC article.
-
Role of the stromal microenvironment in carcinogenesis of the prostate.Int J Cancer. 2003 Oct 20;107(1):1-10. doi: 10.1002/ijc.11335. Int J Cancer. 2003. PMID: 12925950 Review.
-
Vitamin D and benign prostatic hyperplasia -- a review.Can J Urol. 2013 Aug;20(4):6820-5. Can J Urol. 2013. PMID: 23930605 Review.
Cited by
-
The pathogenesis of benign prostatic hyperplasia and the roles of Prdx3, oxidative stress, pyroptosis and autophagy:a review.Front Oncol. 2025 Aug 5;15:1579539. doi: 10.3389/fonc.2025.1579539. eCollection 2025. Front Oncol. 2025. PMID: 40837016 Free PMC article. Review.
-
Novel role of LLGL2 silencing in autophagy: reversing epithelial-mesenchymal transition in prostate cancer.Biol Res. 2024 May 8;57(1):25. doi: 10.1186/s40659-024-00499-w. Biol Res. 2024. PMID: 38720397 Free PMC article.
-
Autophagy in benign prostatic hyperplasia: insights and therapeutic potential.BMC Urol. 2024 Sep 12;24(1):198. doi: 10.1186/s12894-024-01585-7. BMC Urol. 2024. PMID: 39261818 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

