Autologous Platelet-Rich Growth Factor Reduces M1 Macrophages and Modulates Inflammatory Microenvironments to Promote Sciatic Nerve Regeneration
- PMID: 36009539
- PMCID: PMC9406033
- DOI: 10.3390/biomedicines10081991
Autologous Platelet-Rich Growth Factor Reduces M1 Macrophages and Modulates Inflammatory Microenvironments to Promote Sciatic Nerve Regeneration
Abstract
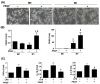
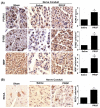
The failure of peripheral nerve regeneration is often associated with the inability to generate a permissive molecular and cellular microenvironment for nerve repair. Autologous therapies, such as platelet-rich plasma (PRP) or its derivative platelet-rich growth factors (PRGF), may improve peripheral nerve regeneration via unknown mechanistic roles and actions in macrophage polarization. In the current study, we hypothesize that excessive and prolonged inflammation might result in the failure of pro-inflammatory M1 macrophage transit to anti-inflammatory M2 macrophages in large nerve defects. PRGF was used in vitro at the time the unpolarized macrophages (M0) macrophages were induced to M1 macrophages to observe if PRGF altered the secretion of cytokines and resulted in a phenotypic change. PRGF was also employed in the nerve conduit of a rat sciatic nerve transection model to identify alterations in macrophages that might influence excessive inflammation and nerve regeneration. PRGF administration reduced the mRNA expression of tumor necrosis factor-α (TNFα), interleukin-1β (IL-1β), and IL-6 in M0 macrophages. Increased CD206 substantiated the shift of pro-inflammatory cytokines to the M2 regenerative macrophage. Administration of PRGF in the nerve conduit after rat sciatic nerve transection promoted nerve regeneration by improving nerve gross morphology and its targeted gastrocnemius muscle mass. The regenerative markers were increased for regrown axons (protein gene product, PGP9.5), Schwann cells (S100β), and myelin basic protein (MBP) after 6 weeks of injury. The decreased expression of TNFα, IL-1β, IL-6, and CD68+ M1 macrophages indicated that the inflammatory microenvironments were reduced in the PRGF-treated nerve tissue. The increase in RECA-positive cells suggested the PRGF also promoted angiogenesis during nerve regeneration. Taken together, these results indicate the potential role and clinical implication of autologous PRGF in regulating inflammatory microenvironments via macrophage polarization after nerve transection.
Keywords: macrophage polarization; peripheral nerve regeneration; platelet-rich growth factors; sciatic nerve injury.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

