Biomarkers of Clot Activation and Degradation and Risk of Future Major Cardiovascular Events in Acute Exacerbation of COPD: A Cohort Sub-Study in a Randomized Trial Population
- PMID: 36009558
- PMCID: PMC9405886
- DOI: 10.3390/biomedicines10082011
Biomarkers of Clot Activation and Degradation and Risk of Future Major Cardiovascular Events in Acute Exacerbation of COPD: A Cohort Sub-Study in a Randomized Trial Population
Abstract
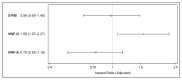
Cardiovascular diseases are common in patients with chronic obstructive pulmonary disease (COPD). Clot formation and resolution secondary to systemic inflammation may be a part of the explanation. The aim was to determine whether biomarkers of clot formation (products of von Willebrand Factor formation and activation) and clot resolution (product of fibrin degeneration) during COPD exacerbation predicted major cardiovascular events (MACE). The cohort was based on clinical data and biobank plasma samples from a trial including patients admitted with an acute exacerbation of COPD (CORTICO-COP). Neo-epitope biomarkers of formation and the activation of von Willebrand factor (VWF-N and V-WFA, respectively) and cross-linked fibrin degradation (X-FIB) were assessed using ELISAs in EDTA plasma at the time of acute admission, and analyzed for time-to-first MACE within 36 months, using multivariable Cox proportional hazards models. In total, 299/318 participants had samples available for analysis. The risk of MACE for patients in the upper quartile of each biomarker versus the lower quartile was: X-FIB: HR 0.98 (95% CI 0.65-1.48), VWF-N: HR 1.56 (95% CI 1.07-2.27), and VWF-A: HR 0.78 (95% CI 0.52-1.16). Thus, in COPD patients with an acute exacerbation, VWF-N was associated with future MACE and warrants further studies in a larger population.
Keywords: COPD exacerbation; biomarkers; coagulation; cross-linked fibrin degradation; major cardiovascular events; von Willebrand factor.
Conflict of interest statement
Authors J.M.B.S., S.R.R., D.J.L. and M.K. are employees and shareholders of Nordic Bioscience. Outside the submitted work: C.S.U. has received grants from Sanofi, Boehringer Ingelheim, AstraZeneca and Novartis; speaker fees from Orion Pharma, AstraZeneca and TEVA; consulting fees from Chiesi, Orion Pharma, AstraZeneca, GSK and TEVA; and has been on advisory boards for Novartis, Sanofi, Glaxo-Smith Kline, Chiesi, AstraZeneca and Boehringer Ingelheim. M.M. has received a grant from Grifols and consulting fees from AstraZeneca, Atriva Therapeutics, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Bial, Gebro Pharma, CSL Behring, Laboratorios Esteve, Ferrer, Mereo Biopharma, Verona Pharma, Spin Therapeutics, pH Pharma, ONO Pharma, Palobiofarma SL, Takeda, Novartis, Sanofi and Grifols; speaker fees from AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, Chiesi, Cipla, Kamada, Takeda, Menarini, Rovi, Bial, Sandoz, Zambon, CSL Behring, Grifols and Novartis; support for attending meetings/travel from Novartis, Boehringer Ingelheim and Menarini; and participation on DSMB for Mereo. EB has received speaker fees from Boehringer Ingelheim and Chiesi, support for attending meetings/travel from Boehringer Ingelheim, and participation on DSMB or advisory board for Boehringer Ingelheim. AB is the secretary of Assembly 5, Airway diseases, Asthma, COPD and Chronic Cough; European Respiratory Society. J.V. has received a grant from Boehringer Ingelheim UK; consulting fees from AstraZeneca, ALK Abello, Boehringer Ingelheim, GSK, Novartis and TEVA; speaker fees from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK and Novartis; and participation on DSMB/advisory boards for AstraZeneca and GSK. All other authors report no conflict of interest. TB-S received consulting fees from GSK and Sanofi Pasteur; received speaker payments from Bayer, Sanofi Pasteur and GSK; support for meetings/travel from AstraZeneca; and received equipment for his department from GE.
Figures

Similar articles
-
Acute exacerbations of COPD are associated with a prothrombotic state through platelet-monocyte complexes, endothelial activation and increased thrombin generation.Respir Med. 2020 Sep;171:106094. doi: 10.1016/j.rmed.2020.106094. Epub 2020 Jul 25. Respir Med. 2020. PMID: 32758992
-
Increased von Willebrand Factor Processing in COPD, Reflecting Lung Epithelium Damage, Is Associated with Emphysema, Exacerbations and Elevated Mortality Risk.Int J Chron Obstruct Pulmon Dis. 2020 Mar 9;15:543-552. doi: 10.2147/COPD.S235673. eCollection 2020. Int J Chron Obstruct Pulmon Dis. 2020. PMID: 32210548 Free PMC article.
-
The Relationship Between Cardiac Troponin in People Hospitalised for Exacerbation of COPD and Major Adverse Cardiac Events (MACE) and COPD Readmissions.Int J Chron Obstruct Pulmon Dis. 2023 Nov 6;18:2405-2416. doi: 10.2147/COPD.S432166. eCollection 2023. Int J Chron Obstruct Pulmon Dis. 2023. PMID: 37955026 Free PMC article.
-
Prognostic value of plasma von Willebrand factor levels in major adverse cardiovascular events: a systematic review and meta-analysis.BMC Cardiovasc Disord. 2020 Feb 10;20(1):72. doi: 10.1186/s12872-020-01375-7. BMC Cardiovasc Disord. 2020. PMID: 32039706 Free PMC article.
-
Association of COPD exacerbations and acute cardiovascular events: a systematic review and meta-analysis.Ther Adv Respir Dis. 2022 Jan-Dec;16:17534666221113647. doi: 10.1177/17534666221113647. Ther Adv Respir Dis. 2022. PMID: 35894441 Free PMC article.
References
-
- Agusti A., Edwards L.D., Rennard S.I., MacNee W., Tal-Singer R., Miller B.E., Vestbo J., Lomas D.A., Calverley P.M., Wouters E., et al. Persistent systemic inflammation is associated with poor clinical outcomes in COPD: A novel phenotype. PLoS ONE. 2012;7:e37483. doi: 10.1371/journal.pone.0037483. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

