Could Weaning Remodel the Oral Microbiota Composition in Donkeys? An Exploratory Study
- PMID: 36009615
- PMCID: PMC9404433
- DOI: 10.3390/ani12162024
Could Weaning Remodel the Oral Microbiota Composition in Donkeys? An Exploratory Study
Abstract
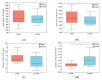
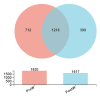
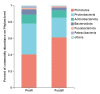
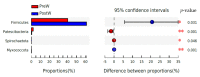
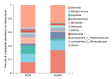
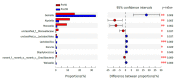
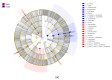
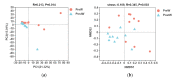
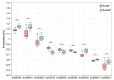
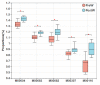
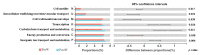
As the initiation point of digestion, the oral microbiome is important in maintaining oral and systemic health. However, the composition of oral microbial communities and the influence of weaning on the oral microbiota of donkey foals remains poorly characterized. The present study used buccal swab samples to determine the changes in oral microbial communities occurring at the time of weaning. A total of 20 oral swab samples were collected from two groups: preweaning donkey foals (PreW group, n = 10) and postweaning donkey foals (PostW group, n = 10). The donkey oral microbiome was analyzed by 16S rRNA gene sequencing using Illumina MiSeq. This study is the first report of the donkey oral microbiome in association with weaning. Compared to the preweaning donkeys, the oral bacteria diversity in the postweaning donkeys was increased, with a higher Simpson index. Changes in the composition of the oral microbiota between the PreW and PostW groups were observed in the present study. At the phylum level, the relative abundance of Firmicutes and Myxococcota was significantly greater in the PostW than in the PreW group. At the genus level, the Gemella, unclassified_o__Lactobacillales, and Lactobacillus were increased in the postweaning donkeys. The donkeys' oral microbial functions were predicted using PICRUSt, and the functions related to carbohydrate metabolic pathways were significantly enriched in the oral microbiome in the PostW donkeys. In summary, the current study provides a deeper insight into the oral microbiota changes during the weaning period, and the influence of weaning together with the documented changes in diversity and composition will help us to obtain a better understanding of their long-term health impact within the oral cavities of donkey foals. However, a major limitation of the present study was that the samples were obtained from different animals in the PreW and PostW groups, which may have resulted in variability among the different individuals. Further investigation is needed to monitor the shift in oral microbes in the same individuals during the weaning period.
Keywords: 16S rRNA; donkey foal; microbial function; oral microbiome; weaning.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Advances in Donkey Disease Surveillance and Microbiome Characterization in China.Microorganisms. 2025 Mar 26;13(4):749. doi: 10.3390/microorganisms13040749. Microorganisms. 2025. PMID: 40284586 Free PMC article. Review.
-
A review of genetic resources and trends of omics applications in donkey research: focus on China.Front Vet Sci. 2024 Oct 11;11:1366128. doi: 10.3389/fvets.2024.1366128. eCollection 2024. Front Vet Sci. 2024. PMID: 39464628 Free PMC article. Review.
-
Dynamic changes in fecal microbiota in donkey foals during weaning: From pre-weaning to post-weaning.Front Microbiol. 2023 Jan 27;14:1105330. doi: 10.3389/fmicb.2023.1105330. eCollection 2023. Front Microbiol. 2023. PMID: 36778861 Free PMC article.
-
Comprehensive omics analysis of the fecal microbiome and serum metabolome in Dezhou donkey foals at the end of weaning and after weaning.Anim Sci J. 2025 Jan-Dec;96(1):e70021. doi: 10.1111/asj.70021. Anim Sci J. 2025. PMID: 39815660
-
Metabolic changes before and after weaning in Dezhou donkey foals in relation to gut microbiota.Front Microbiol. 2024 Jan 12;14:1306039. doi: 10.3389/fmicb.2023.1306039. eCollection 2023. Front Microbiol. 2024. PMID: 38282742 Free PMC article.
Cited by
-
A survey report on the donkey original breeding farms in China: Current aspects and future prospective.Front Vet Sci. 2023 Mar 16;10:1126138. doi: 10.3389/fvets.2023.1126138. eCollection 2023. Front Vet Sci. 2023. PMID: 37008357 Free PMC article.
-
Periodontitis Disease in Farmed Ruminants-Current State of Research.Int J Mol Sci. 2023 Jun 5;24(11):9763. doi: 10.3390/ijms24119763. Int J Mol Sci. 2023. PMID: 37298712 Free PMC article. Review.
-
Advances in Donkey Disease Surveillance and Microbiome Characterization in China.Microorganisms. 2025 Mar 26;13(4):749. doi: 10.3390/microorganisms13040749. Microorganisms. 2025. PMID: 40284586 Free PMC article. Review.
-
A review of genetic resources and trends of omics applications in donkey research: focus on China.Front Vet Sci. 2024 Oct 11;11:1366128. doi: 10.3389/fvets.2024.1366128. eCollection 2024. Front Vet Sci. 2024. PMID: 39464628 Free PMC article. Review.
-
Exploring the Effect of Gastrointestinal Prevotella on Growth Performance Traits in Livestock Animals.Animals (Basel). 2024 Jul 2;14(13):1965. doi: 10.3390/ani14131965. Animals (Basel). 2024. PMID: 38998077 Free PMC article. Review.
References
-
- Arfuso F., Bazzano M., Brianti E., Gaglio G., Passantino A., Tesei B., Laus F. Nutritional supplements containing Cardus mariano, Eucalyptus globulus, Gentiana lutea, Urtica urens, and Mallotus philippinensis extracts are effective in reducing egg shedding in dairy jennies (Equus asinus) naturallyinfected by cyathostomins. Front. Vet. Sci. 2020;7:556270. doi: 10.3389/fvets.2020.556270. - DOI - PMC - PubMed
-
- Piccione G., Assenza A., Costa A., Fazio F., Caola G. Daily rhythms of the body temperature and some haematochemical parameters in donkey. Slov. Vet. Res. 2003;40:71–76.
Grants and funding
- SDAIT-27/Shandong Province Modern Agricultural Technology System Donkey Industrial Innovation Team
- 19211162/The Livestock and Poultry Breeding Industry Project of the Ministry of Agriculture and Rural Affairs
- 2021TZXD012/The Key R & D project of Shandong Province
- 319312101-14/The Open Project of Liaocheng University Animal Husbandry Discipline
- 3193308/The Open Project of Shandong Collaborative Innovation Center for Donkey Industry Technology
LinkOut - more resources
Full Text Sources
Research Materials

