Effects of Casein Phosphopeptide-Selenium Complex on the Immune Functions in Beagle Dogs
- PMID: 36009627
- PMCID: PMC9404450
- DOI: 10.3390/ani12162037
Effects of Casein Phosphopeptide-Selenium Complex on the Immune Functions in Beagle Dogs
Abstract
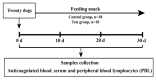
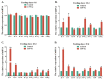
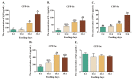
The health of pets is becoming a growing concern for the pet industry and its owners. Immunity is one of the foundational supports for health, thus developing a functional bioactive substance that can boost pets' immunity is essential. Many studies have shown that casein phosphopeptide (CPP) and selenium (Se) can individually regulate immunity in many species, but there has been no reported research on the immunomodulatory function of casein phosphopeptide-selenium complex (CPP-Se). The objective of this study was to investigate the function of CPP-Se on immunomodulation in dogs. Twenty Beagle dogs were equally divided into two groups and fed either a control snack or a test snack supplemented with 0.03% CPP-Se for 30 days. Anticoagulated blood, serum and peripheral blood lymphocytes (PBL) were collected from dogs at 0 d, 10 d, 20 d and 30 d to detect the change in the number of immune cells and the expression of cytokine-related mRNAs and proteins. PBL isolated from blood were exposed to CPP-Se in vitro to measure the proliferative responses and cytokine-related mRNAs expression. During the time the test snack was fed, the number of lymphocytes increased significantly, whereas neutrophils and monocytes remained unaltered. The expression of interleukin-4 (IL-4), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), CD4 molecule (CD4) and CD8α molecule (CD8α) was up-regulated, while interleukin-1β (IL-1β) was down-regulated, and interleukin-10 (IL-10) declined initially and subsequently increased. ELISA detection revealed a significant increment in serum IL-4, IL-6, Immunoglobulin M (IgM) and IFN-γ, except for IgG. Furthermore, CPP-Se treatment increased the proliferation and the expression of cytokine-related mRNAs in PBL cultured in vitro. This is the first study to demonstrate that CPP-Se can improve immunity in the dog.
Keywords: casein phosphopeptide–selenium complex (CPP-Se); cytokines; dogs; immunity; lymphocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Sheikh A.B., Javed N., Leyba K., Khair A.H., Ijaz Z., Dar A.A., Hanif H., Farooq A., Shekhar R. Pet-assisted therapy for delirium and agitation in hospitalized patients with neurocognitive impairment: A review of literature. Geriatrics. 2021;6:96. doi: 10.3390/geriatrics6040096. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

