Expression of Exogenous GFP- CesA6 in Tobacco Enhances Cell Wall Biosynthesis and Biomass Production
- PMID: 36009766
- PMCID: PMC9405164
- DOI: 10.3390/biology11081139
Expression of Exogenous GFP- CesA6 in Tobacco Enhances Cell Wall Biosynthesis and Biomass Production
Abstract
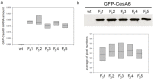
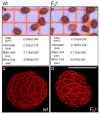
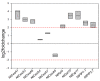
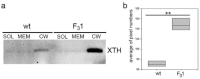
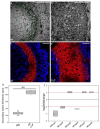
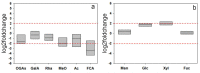
Improved cellulose biosynthesis and plant biomass represent important economic targets for several biotechnological applications including bioenergy and biofuel production. The attempts to increase the biosynthesis of cellulose by overexpressing CesAs proteins, components of the cellulose synthase complex, has not always produced consistent results. Analyses of morphological and molecular data and of the chemical composition of cell walls showed that tobacco plants (F31 line), stably expressing the Arabidopsis CesA6 fused to GFP, exhibits a "giant" phenotype with no apparent other morphological aberrations. In the F31 line, all evaluated growth parameters, such as stem and root length, leaf size, and lignified secondary xylem, were significantly higher than in wt. Furthermore, F31 line exhibited increased flower and seed number, and an advance of about 20 days in the anthesis. In the leaves of F31 seedlings, the expression of primary CesAs (NtCesA1, NtCesA3, and NtCesA6) was enhanced, as well as of proteins involved in the biosynthesis of non-cellulosic polysaccharides (xyloglucans and galacturonans, NtXyl4, NtGal10), cell wall remodeling (NtExp11 and XTHs), and cell expansion (NtPIP1.1 and NtPIP2.7). While in leaves the expression level of all secondary cell wall CesAs (NtCesA4, NtCesA7, and NtCesA8) did not change significantly, both primary and secondary CesAs were differentially expressed in the stem. The amount of cellulose and matrix polysaccharides significantly increased in the F31 seedlings with no differences in pectin and hemicellulose glycosyl composition. Our results highlight the potentiality to overexpress primary CesAs in tobacco plants to enhance cellulose synthesis and biomass production.
Keywords: AtCesA6; Nicotiana tabacum; cell wall; cellulose; cellulose synthase complex; matrix polysaccharides; plant growth; primary and secondary CesAs.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Brown R.M., Jr. Cellulose structure and biosynthesis: What is in store for the 21st century? J. Polym. Sci. Part A Polym. Chem. 2004;42:487–495. doi: 10.1002/pola.10877. - DOI
-
- Desprez T., Juraniec M., Crowell E.F., Jouy H., Pochylova Z., Parcy F., Höfte H., Gonneau M., Vernetthes S. Organization of cellulose synthase complexes involved in primary cell wall synthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2007;104:15572–15577. doi: 10.1073/pnas.0706569104. - DOI - PMC - PubMed
-
- Rodriguez-Restrepo Y.A., Rocha C.M.R., Teixeira J.A., Orrego C.E. Valorization of Passion Fruit Stalk by the Preparation of Cellulose Nanofibers and Immobilization of Trypsin. Fibers. Polym. 2020;21:2807–2816. doi: 10.1007/s12221-020-1342-2. - DOI
LinkOut - more resources
Full Text Sources

