Thermostable and O2-Insensitive Pyruvate Decarboxylases from Thermoacidophilic Archaea Catalyzing the Production of Acetaldehyde
- PMID: 36009875
- PMCID: PMC9405506
- DOI: 10.3390/biology11081247
Thermostable and O2-Insensitive Pyruvate Decarboxylases from Thermoacidophilic Archaea Catalyzing the Production of Acetaldehyde
Abstract
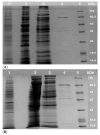
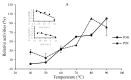
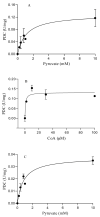
Pyruvate decarboxylase (PDC) is a key enzyme involved in ethanol fermentation, and it catalyzes the decarboxylation of pyruvate to acetaldehyde and CO2. Bifunctional PORs/PDCs that also have additional pyruvate:ferredoxin oxidoreductase (POR) activity are found in hyperthermophiles, and they are mostly oxygen-sensitive and CoA-dependent. Thermostable and oxygen-stable PDC activity is highly desirable for biotechnological applications. The enzymes from the thermoacidophiles Saccharolobus (formerly Sulfolobus) solfataricus (Ss, Topt = 80 °C) and Sulfolobus acidocaldarius (Sa, Topt = 80 °C) were purified and characterized, and their biophysical and biochemical properties were determined comparatively. Both enzymes were shown to be heterodimeric, and their two subunits were determined by SDS-PAGE to be 37 ± 3 kDa and 65 ± 2 kDa, respectively. The purified enzymes from S. solfataricus and S. acidocaldarius showed both PDC and POR activities which were CoA-dependent, and they were thermostable with half-life times of 2.9 ± 1 and 1.1 ± 1 h at 80 °C, respectively. There was no loss of activity in the presence of oxygen. Optimal pH values for their PDC and POR activity were determined to be 7.9 and 8.6, respectively. In conclusion, both thermostable SsPOR/PDC and SaPOR/PDC catalyze the CoA-dependent production of acetaldehyde from pyruvate in the presence of oxygen.
Keywords: Saccharolobus solfataricus; Sulfolobus acidocaldarius; archaea; ethanol fermentation; hyperthermophiles; pyruvate decarboxylase; pyruvate:ferredoxin oxidoreductase.
Conflict of interest statement
The authors declare that they have no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures





Similar articles
-
Decarboxylation of pyruvate to acetaldehyde for ethanol production by hyperthermophiles.Biomolecules. 2013 Aug 21;3(3):578-96. doi: 10.3390/biom3030578. Biomolecules. 2013. PMID: 24970182 Free PMC article.
-
Molecular and biochemical characterization of bifunctional pyruvate decarboxylases and pyruvate ferredoxin oxidoreductases from Thermotoga maritima and Thermotoga hypogea.J Biochem. 2015 Dec;158(6):459-66. doi: 10.1093/jb/mvv058. Epub 2015 Jun 1. J Biochem. 2015. PMID: 26032540
-
The bifunctional pyruvate decarboxylase/pyruvate ferredoxin oxidoreductase from Thermococcus guaymasensis.Archaea. 2014 May 29;2014:349379. doi: 10.1155/2014/349379. eCollection 2014. Archaea. 2014. PMID: 24982594 Free PMC article.
-
The pyruvate dehydrogenase complex from thermophilic organisms: thermal stability and re-association from the enzyme components.Biochim Biophys Acta. 1998 Jun 29;1385(2):341-52. doi: 10.1016/s0167-4838(98)00078-8. Biochim Biophys Acta. 1998. PMID: 9655930 Review.
-
[Vitamin B1].Nihon Rinsho. 1999 Oct;57(10):2187-92. Nihon Rinsho. 1999. PMID: 10540860 Review. Japanese.
References
-
- Tse T.J., Wiens D.J., Reaney J.T. Production of bioethanol—A review of factors affecting ethanol yield. Fermentation. 2021;7:268. doi: 10.3390/fermentation7040268. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

