Assessment of the Impact of a Meningitis/Encephalitis Panel on Hospital Length of Stay: A Systematic Review and Meta-Analysis
- PMID: 36009898
- PMCID: PMC9405449
- DOI: 10.3390/antibiotics11081028
Assessment of the Impact of a Meningitis/Encephalitis Panel on Hospital Length of Stay: A Systematic Review and Meta-Analysis
Abstract
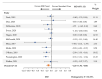
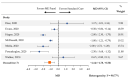
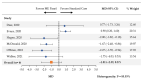
Meningitis and encephalitis are central nervous system infections with considerable morbidity and mortality. The BioFire® FilmArray® Meningitis/Encephalitis Panel (multiplex ME panel) can identify pathogens rapidly potentially aiding in targeted therapy and curtail antimicrobial exposure. This systematic review and meta-analysis synthesized the literature on the association between the multiplex ME panel and length of hospital stay (LOS), length of acyclovir therapy, and days with antibiotics. MEDLINE and EMBASE were searched. Only studies presenting novel data were retained. Random-effects meta-analyses were performed to assess the impact of the multiplex ME panel on outcomes. Of 169 retrieved publications, 13 met the criteria for inclusion. Patients tested with the multiplex ME panel had a reduction in the average LOS (mean difference [MD] [95% CI]: -1.20 days [-1.96, -0.44], n = 11 studies). Use of the multiplex ME panel was also associated with a reduction in the length of acyclovir therapy (MD [95% CI]: -1.14 days [-1.78, -0.50], n = 7 studies) and a nonsignificant reduction in the average number of days with antibiotics (MD [95% CI]: -1.01 days [-2.39, 0.37], n = 6 studies). The rapidity of pathogen identification contributes to an overall reduced LOS, reductions in the duration of empiric antiviral utilization, and a nonsignificant reduction in antibiotic therapy.
Keywords: diagnostic techniques; encephalitis; meningitis; neurological; patient care; polymerase chain reaction.
Conflict of interest statement
N.K., P.T.-L., T.I.T. and K.M. are employees of Analysis Group, Inc. (Boston, MA, USA), a consulting company that has received research funding from BioFire Diagnostics, L.L.C. (Salt Lake City, UT, USA), for the conduct of this study. K.D.H. and T.T.T. are employees of BioFire Diagnostics, L.L.C. BioFire Diagnostics, L.L.C. was involved in the study design, data collection, data analysis, manuscript preparation, and publication decisions. R.H has received research support and personal fees from BioFire Diagnostics, L.L.C.
Figures
References
-
- Greenlee J.E. Acute Bacterial Meningitis. Medical Topics & Chapters, Volume 2021. Merck Manual Professional Version, 2020. [(accessed on 21 July 2022)]. Available online: https://www.msdmanuals.com/en-sg/home/brain,-spinal-cord,-and-nerve-diso....
-
- Tunkel A.R., Glaser C.A., Bloch K.C., Sejvar J.J., Marra C.M., Roos K.L., Hartman B.J., Kaplan S.L., Scheld W.M., Whitley R.J. The Management of Encephalitis: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2008;47:303–327. doi: 10.1086/589747. - DOI - PubMed
-
- Zunt J.R., Kassebaum N.J., Blake N., Glennie L., Wright C., Nichols E., Abd-Allah F., Abdela J., Abdelalim A., Adamu A.A., et al. Global, regional, and national burden of meningitis, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:1061–1082. doi: 10.1016/S1474-4422(18)30387-9. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

