Model-Informed Translation of In Vitro Effects of Short-, Prolonged- and Continuous-Infusion Meropenem against Pseudomonas aeruginosa to Clinical Settings
- PMID: 36009905
- PMCID: PMC9404958
- DOI: 10.3390/antibiotics11081036
Model-Informed Translation of In Vitro Effects of Short-, Prolonged- and Continuous-Infusion Meropenem against Pseudomonas aeruginosa to Clinical Settings
Abstract
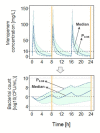
Pharmacokinetic-pharmacodynamic (PKPD) models have met increasing interest as tools to identify potential efficacious antibiotic dosing regimens in vitro and in vivo. We sought to investigate the impact of diversely shaped clinical pharmacokinetic profiles of meropenem on the growth/killing patterns of Pseudomonas aeruginosa (ARU552, MIC = 16 mg/L) over time using a semi-mechanistic PKPD model and a PK/PD index-based approach. Bacterial growth/killing were driven by the PK profiles of six patient populations (infected adults, burns, critically ill, neurosurgery, obese patients) given varied pathogen features (e.g., EC50, growth rate, inoculum), patient characteristics (e.g., creatinine clearance), and ten dosing regimens (including two dose levels and 0.5-h, 3-h and continuous-infusion regimens). Conclusions regarding the most favourable dosing regimen depended on the assessment of (i) the total bacterial load or fT>MIC (time that unbound concentrations exceed the minimum inhibitory concentration); (ii) the median or P0.95 profile of the population; and (iii) 8 h or 24 h time points. Continuous infusion plus loading dose as well as 3-h infusions (3-h infusions: e.g., for scenarios associated with low meropenem concentrations, P0.95 profiles, and MIC ≥ 16 mg/L) appeared superior to standard 0.5-h infusions at 24 h. The developed platform can serve to identify promising strategies of efficacious dosing for clinical trials.
Keywords: PK/PD index; PKPD model; Pseudomonas aeruginosa; beta-lactam; continuous infusion; meropenem; pharmacometrics; prolonged infusion; time-kill curve.
Conflict of interest statement
The authors declare that they have no conflict of interest that are relevant to the content of this article.
Figures


References
-
- Tacconelli E., Carrara E., Savoldi A., Harbarth S., Mendelson M., Monnet D.L., Pulcini C., Kahlmeter G., Kluytmans J., Carmeli Y., et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018;18:318–327. doi: 10.1016/S1473-3099(17)30753-3. - DOI - PubMed
-
- AstraZeneca Pharmaceuticals LP Merrem® (Meropenem for Injection and for Intravenous Use)—Summary of Product Characteristics 2016. [(accessed on 10 June 2022)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/050706s037lbl.pdf.
-
- Lipman J., Brett S.J., De Waele J.J., Cotta M.O., Davis J.S., Finfer S., Glass P., Knowles S., McGuinness S., Myburgh J., et al. A Protocol for a Phase 3 Multicentre Randomised Controlled Trial of Continuous versus Intermittent β-Lactam Antibiotic Infusion in Critically Ill Patients with Sepsis: BLING III. Crit. Care Resusc. 2019;21:63–68. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

