Development and Characterization of Azithromycin-Loaded Microemulsions: A Promising Tool for the Treatment of Bacterial Skin Infections
- PMID: 36009909
- PMCID: PMC9404999
- DOI: 10.3390/antibiotics11081040
Development and Characterization of Azithromycin-Loaded Microemulsions: A Promising Tool for the Treatment of Bacterial Skin Infections
Abstract
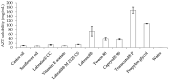
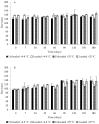
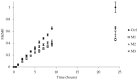
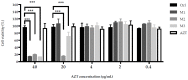
In recent years, the treatment of bacterial skin infections has been considered a major healthcare issue due to the growing emergence of antibiotic-resistant strains of Staphylococcus aureus. The incorporation of antibiotics in appropriate nanosystems could represent a promising strategy, able to overcome several drawbacks of the topical treatment of infections, including poor drug retention within the skin. The present work aims to develop microemulsions containing azithromycin (AZT), a broad-spectrum macrolide antibiotic. Firstly, AZT solubility in various oils, surfactants and co-surfactants was assessed to select the main components. Subsequently, microemulsions composed of vitamin E acetate, Labrasol® and Transcutol® P were prepared and characterized for their pH, viscosity, droplet size, zeta potential and ability to release the drug and to promote its retention inside porcine skin. Antimicrobial activity against S. aureus methicillin-resistant strains (MRSA) and the biocompatibility of microemulsions were evaluated. Microemulsions showed an acceptable pH and were characterized by different droplet sizes and viscosities depending on their composition. Interestingly, they provided a prolonged release of AZT and promoted its accumulation inside the skin. Finally, microemulsions retained AZT efficacy on MRSA and were not cytotoxic. Hence, the developed AZT-loaded microemulsions could be considered as useful nanocarriers for the treatment of antibiotic-resistant infections of the skin.
Keywords: azithromycin; methicillin-resistant Staphylococcus aureus; microemulsions; permeation/retention studies; skin infections; topical delivery.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Tognetti L., Martinelli C., Berti S., Hercogova J., Lotti T., Leoncini F., Moretti S. Bacterial skin and soft tissue infections: Review of the epidemiology, microbiology, aetiopathogenesis and treatment: A collaboration between dermatologists and infectivologists. J. Eur. Acad. Dermatol. Venereol. 2012;26:931–941. doi: 10.1111/j.1468-3083.2011.04416.x. - DOI - PubMed
-
- Ferreira M., Ogren M., Dias J.N.R., Silva M., Gil S., Tavares L., Aires-da-Silva F., Gaspar M.M., Aguiar S.I. Liposomes as Antibiotic Delivery Systems: A Promising Nanotechnological Strategy against Antimicrobial Resistance. Molecules. 2021;26:2047. doi: 10.3390/molecules26072047. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

