Outpatient Antibiotic and Antiviral Utilization Patterns in Patients Tested for Respiratory Pathogens in the United States: A Real-World Database Study
- PMID: 36009927
- PMCID: PMC9405217
- DOI: 10.3390/antibiotics11081058
Outpatient Antibiotic and Antiviral Utilization Patterns in Patients Tested for Respiratory Pathogens in the United States: A Real-World Database Study
Abstract
This retrospective observational study evaluated outpatient treatment patterns among patients with molecular-based viral diagnostic testing for suspected upper respiratory tract infections in the United States. Patients with a respiratory viral test were identified from 1 August 2016 to 1 July 2019 in a large national reference laboratory database linked to IQVIA's prescription and medical claims databases. Antibiotic and influenza antiviral treatment patterns were reported up to 7 days post-test result. Predictors of antibiotic utilization were assessed using multivariable logistic regression. Among 9561 patients included in the study, 24.6% had evidence of ≥1 filled antibiotic prescription. Antibiotic utilization was higher in patients who tested negative for all viral targets (odds ratio [OR], 1.33; 95% confidence interval [CI], 1.17-1.50) and patients positive for non-influenza viruses (OR, 1.28; 95% CI, 1.09-1.51) compared with those influenza-positive only. Age ≥ 50 years and location outside of the northeast United States also predicted antibiotic utilization. Influenza antivirals were more common in influenza-positive patients compared with patients with other test results (32.5% vs. 3.6-9.0%). Thus, in this real-world study, antibiotic utilization was elevated in patients positive for non-influenza viruses, although antibiotics would generally not be indicated. Further research on pairing diagnostic tools with outpatient antibiotic stewardship programs is needed.
Keywords: antibiotic; antiviral; diagnostics; outpatients; real-world; respiratory tract infections; stewardship.
Conflict of interest statement
James Karichu, Brian Lee, and Susan N. Chang are employed by Roche Diagnostics Solutions. Mindy Cheng was formerly an employee of Roche Diagnostics Solutions at the time this study was conducted. Jenny Tse and Aimee M. Near are employees of IQVIA, which was paid by Roche Diagnostics Solutions to conduct the data analysis and manuscript writing.
Figures
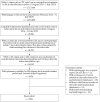

Similar articles
-
Outpatient Antibiotic Prescribing for Acute Respiratory Infections During Influenza Seasons.JAMA Netw Open. 2018 Jun 1;1(2):e180243. doi: 10.1001/jamanetworkopen.2018.0243. JAMA Netw Open. 2018. PMID: 30646067 Free PMC article.
-
The Impact of Rapid Molecular Diagnostics for Influenza on Antibiotic Stewardship in the Emergency Department-An Observational Retrospective Study.Antibiotics (Basel). 2025 Jan 23;14(2):120. doi: 10.3390/antibiotics14020120. Antibiotics (Basel). 2025. PMID: 40001364 Free PMC article.
-
Burden of influenza in patients with cardiovascular disease who receive antiviral treatment for influenza.J Med Econ. 2022 Jan-Dec;25(1):1061-1067. doi: 10.1080/13696998.2022.2111910. J Med Econ. 2022. PMID: 35943115
-
Antibiotic prescriptions associated with outpatient visits for acute upper respiratory tract infections among adult Medicaid recipients in North Carolina.N C Med J. 2003 Jul-Aug;64(4):148-56. N C Med J. 2003. PMID: 12971239
-
Impact of multiplexed respiratory viral panels on infection control measures and antimicrobial stewardship: a review of the literature.Eur J Clin Microbiol Infect Dis. 2022 Feb;41(2):187-202. doi: 10.1007/s10096-021-04375-3. Epub 2021 Nov 20. Eur J Clin Microbiol Infect Dis. 2022. PMID: 34799754 Free PMC article. Review.
Cited by
-
Characteristics of initiators of budesonide/glycopyrrolate/formoterol for treatment of chronic obstructive pulmonary disease (COPD) in the United States: the AURA study.Ther Adv Respir Dis. 2023 Jan-Dec;17:17534666231164534. doi: 10.1177/17534666231164534. Ther Adv Respir Dis. 2023. PMID: 37013423 Free PMC article.
-
Curtailing virus-induced inflammation in respiratory infections: emerging strategies for therapeutic interventions.Front Pharmacol. 2023 May 5;14:1087850. doi: 10.3389/fphar.2023.1087850. eCollection 2023. Front Pharmacol. 2023. PMID: 37214455 Free PMC article. Review.
-
Point-of-Care and Rapid Tests for the Etiological Diagnosis of Respiratory Tract Infections in Children: A Systematic Review and Meta-Analysis.Antibiotics (Basel). 2022 Sep 3;11(9):1192. doi: 10.3390/antibiotics11091192. Antibiotics (Basel). 2022. PMID: 36139971 Free PMC article. Review.
-
The epidemiology of pediatric outpatient acute respiratory tract infections in the US: a multi-facility analysis of multiplex PCR testing from 2018 to 2023.Microbiol Spectr. 2024 Jan 11;12(1):e0342323. doi: 10.1128/spectrum.03423-23. Epub 2023 Dec 14. Microbiol Spectr. 2024. PMID: 38095469 Free PMC article.
References
-
- Havers F.P., Hicks L.A., Chung J.R., Gaglani M., Murthy K., Zimmerman R.K., Jackson L.A., Petrie J.G., McLean H.Q., Nowalk M.P., et al. Outpatient Antibiotic Prescribing for Acute Respiratory Infections During Influenza Seasons. JAMA Netw. Open. 2018;1:e180243. doi: 10.1001/jamanetworkopen.2018.0243. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

