New Pyrazolo-Benzimidazole Mannich Bases with Antimicrobial and Antibiofilm Activities
- PMID: 36009963
- PMCID: PMC9405415
- DOI: 10.3390/antibiotics11081094
New Pyrazolo-Benzimidazole Mannich Bases with Antimicrobial and Antibiofilm Activities
Abstract
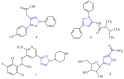
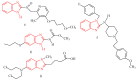
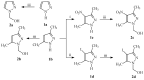
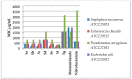
A new series of pyrazolo-benzimidazole hybrid Mannich bases were synthesized, characterized by 1H-NMR, 13C-NMR, IR, UV-Vis, MS, and elemental analysis. In vitro cytotoxicity of the new compounds studied on fibroblast cells showed that the newly synthesized pyrazolo-benzimidazole hybrid derivatives were noncytotoxic until the concentration of 1 μM and two compounds presented a high degree of biocompatibility. The antibacterial and antibiofilm activity of the newly synthesized compounds was assayed on Gram-positive Staphylococcus aureus ATCC25923, Enterococcus faecalis ATCC29212, and Gram-negative Pseudomonas aeruginosa ATCC27853, Escherichia coli ATCC25922 strains. All synthesized compounds 5a-g are more active against all three tested bacterial strains Staphylococcus aureus ATCC25923, Enterococcus faecalis ATCC29212, and Escherichia coli ATCC25922 than reference drugs (Metronidazole, Nitrofurantoin), with the exception of compounds 5d and 5g, which are less active compared to Nitrofurantoin, and all synthesized compounds 5a-g are more active against Pseudomonas aeruginosa ATCC27853 compared to reference drugs (Metronidazole, Nitrofurantoin). Compound 5f showed the best activity against Staphylococcus aureus ATCC 25923, with a MIC of 150 μg/mL and has also inhibited the biofilm formed by all the bacterial strains, having an MBIC of 310 µg/mL compared to the reference drugs (Metronidazole, Nitrofurantoin).
Keywords: antimicrobial; benzimidazoles; biofilm formation; cytotoxicity; hybrid heterocyclic molecules; pyrazoles.
Conflict of interest statement
The authors confirm that this article’s content has no conflict of interest.
Figures






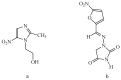

Similar articles
-
Synthesis, Spectroscopic Characterization, Structural Analysis, and Evaluation of Anti-Tumor, Antimicrobial, and Antibiofilm Activities of Halogenoaminopyrazoles Derivatives.Antibiotics (Basel). 2024 Nov 22;13(12):1119. doi: 10.3390/antibiotics13121119. Antibiotics (Basel). 2024. PMID: 39766509 Free PMC article.
-
Synthesis, antibacterial and antifungal activities of electron-rich olefins derived benzimidazole compounds.Farmaco. 2003 Jun;58(6):431-7. doi: 10.1016/S0014-827X(03)00068-5. Farmaco. 2003. PMID: 12767382
-
Antimicrobial and cytotoxic activities of isoniazid connected menthone derivatives and their investigation of clinical pathogens causing infectious disease.J Infect Public Health. 2021 Apr;14(4):533-542. doi: 10.1016/j.jiph.2020.12.033. Epub 2021 Feb 6. J Infect Public Health. 2021. PMID: 33744741
-
Synthesis of some benzimidazole derivatives and their antibacterial and antifungal activities.Arzneimittelforschung. 2001;51(5):420-4. doi: 10.1055/s-0031-1300057. Arzneimittelforschung. 2001. PMID: 11413744
-
Synthesis and antibacterial activities of new bis-benzimidazoles.Arzneimittelforschung. 2004;54(1):64-8. doi: 10.1055/s-0031-1296938. Arzneimittelforschung. 2004. PMID: 14979611
Cited by
-
Design, Synthesis, Molecular Modeling and Biological Evaluation of Novel Pyrazole Benzimidazolone Derivatives as Potent Antioxidants.Pharmaceuticals (Basel). 2023 Nov 24;16(12):1648. doi: 10.3390/ph16121648. Pharmaceuticals (Basel). 2023. PMID: 38139775 Free PMC article.
-
Antibacterial Potential of Novel Acetamide Derivatives of 2-Mercaptobenzothiazole: Synthesis and Docking Studies.ACS Omega. 2023 Mar 9;8(11):9785-9796. doi: 10.1021/acsomega.2c05782. eCollection 2023 Mar 21. ACS Omega. 2023. PMID: 36969428 Free PMC article.
-
Synthesis, Antimicrobial and Antibiofilm Activities, and Molecular Docking Investigations of 2-(1H-Indol-3-yl)-1H-benzo[d]imidazole Derivatives.Molecules. 2023 Oct 14;28(20):7095. doi: 10.3390/molecules28207095. Molecules. 2023. PMID: 37894573 Free PMC article.
-
Synthesis, Spectroscopic Characterization, Structural Analysis, and Evaluation of Anti-Tumor, Antimicrobial, and Antibiofilm Activities of Halogenoaminopyrazoles Derivatives.Antibiotics (Basel). 2024 Nov 22;13(12):1119. doi: 10.3390/antibiotics13121119. Antibiotics (Basel). 2024. PMID: 39766509 Free PMC article.
-
Benzimidazole-Triazole Hybrids as Antimicrobial and Antiviral Agents: A Systematic Review.Antibiotics (Basel). 2023 Jul 22;12(7):1220. doi: 10.3390/antibiotics12071220. Antibiotics (Basel). 2023. PMID: 37508316 Free PMC article. Review.
References
-
- Reddy T.S., Kulhari H., Reddy V.G., Bansal V., Kamal A., Shukla R. Design, synthesis and biological evaluation of 1,3-diphenyl-1H-pyrazole derivatives containing benzimidazole skeleton as potential anticancer and apoptosis inducing agents. Eur. J. Med. Chem. 2015;101:790–805. doi: 10.1016/j.ejmech.2015.07.031. - DOI - PubMed
-
- Marinescu M., Zalaru C.M. Synthesis, Antibacterial and Anti-Tumor Activity of Pyrazole Derivatives. In: MedDocs eBooks, editor. Recent Trends in Biochemistry. MedDocs Publishers LLC; Reno, NV, USA: 2021. [(accessed on 1 January 2020)]. pp. 18–27. Chapter 3. Available online: http://meddocsonline.org/
-
- Caliskan B., Yilmaz A., Evren I., Menevse S., Uludag O., Banoglu E. Synthesis and evaluation of analgesic, anti-inflammatory, and anticancer activities of new pyrazole-3(5)-carboxylic acid derivatives. Med. Chem. Res. 2013;22:782–793. doi: 10.1007/s00044-012-0072-4. - DOI
-
- Gokhan-Kelekci N., Koyunoglu S., Yabanoglu S., Yelekci K., Ozgen O., Ucar G., Erol K., Kendi E., Yesilada A. New pyrazoline bearing, 4(3H)-quinazolinone inhibitors of monoamine oxidase: Synthesis, biological evaluation, and structural determinants of MAO-A and MAO-B selectivity. Bioorg. Med. Chem. 2009;17:675–689. doi: 10.1016/j.bmc.2008.11.068. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

