Chelation in Antibacterial Drugs: From Nitroxoline to Cefiderocol and Beyond
- PMID: 36009974
- PMCID: PMC9405089
- DOI: 10.3390/antibiotics11081105
Chelation in Antibacterial Drugs: From Nitroxoline to Cefiderocol and Beyond
Abstract
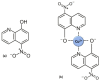
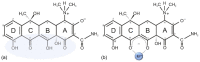
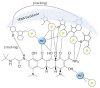
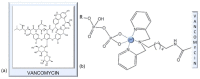
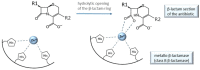
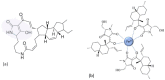
In the era of escalating antimicrobial resistance, the need for antibacterial drugs with novel or improved modes of action (MOAs) is a health concern of utmost importance. Adding or improving the chelating abilities of existing drugs or finding new, nature-inspired chelating agents seems to be one of the major ways to ensure progress. This review article provides insight into the modes of action of antibacterial agents, class by class, through the perspective of chelation. We covered a wide scope of antibacterials, from a century-old quintessential chelating agent nitroxoline, currently unearthed due to its newly discovered anticancer and antibiofilm activities, over the commonly used antibacterial classes, to new cephalosporin cefiderocol and a potential future class of tetramates. We show the impressive spectrum of roles that chelation plays in antibacterial MOAs. This, by itself, demonstrates the importance of understanding the fundamental chemistry behind such complex processes.
Keywords: antibacterial drugs; antibacterial modes of action (MOAs); chelation; nitroxoline.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Barzic A.I., Ioan S. Antibacterial Drugs—From Basic Concepts to Complex Therapeutic Mechanisms of Polymer Systems. In: Bobbarala V., editor. Concepts, Compounds and the Alternatives of Antibacterials. IntechOpen; London, UK: 2015.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

