Both Manuka and Non-Manuka Honey Types Inhibit Antibiotic Resistant Wound-Infecting Bacteria
- PMID: 36010001
- PMCID: PMC9405051
- DOI: 10.3390/antibiotics11081132
Both Manuka and Non-Manuka Honey Types Inhibit Antibiotic Resistant Wound-Infecting Bacteria
Abstract
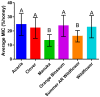
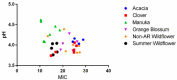
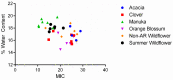
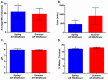
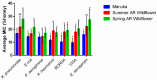
Postoperative infections are a major concern in United States hospitals, accounting for roughly 20% of all hospital-acquired infections yearly. Wound-infecting bacteria, in particular, have a high rate of drug resistance (up to 65%), creating life-threatening complications. Manuka honey, native to New Zealand, has been FDA-approved for wound treatment in the United States after studies demonstrated its ability to inhibit a variety of bacterial species and facilitate wound healing. The aim of this study was to identify alternative (non-manuka) honey types that can be specifically used against antibiotic resistance bacteria in wound infections. We utilized a honey-plate method to measure the minimum inhibitory concentration (MIC) of honey to avoid the limitations of agar diffusion, where large, nonpolar polyphenols (which will not diffuse efficiently) play an important role in bioactivity. This study demonstrated that there are several alternative (non-manuka) honey types, particularly fresh raw Arkansas wildflower honeys, that comparably inhibit the growth of the antibiotic-resistant bacterial species specifically implicated in wound infections. Concentrations of 10-30% honey inhibited the growth of the highly antibiotic-resistant organisms colloquially referred to as "superbugs", which the WHO declared in 2017 to be in critical need of new antibiotics. There was no statistical difference between manuka honey and fresh summer Arkansas wildflower honey in overall bacterial inhibition. These results could transform wound care in the United States, where manuka honey can be expensive and difficult to obtain and where antibiotic resistance remains a troubling concern for wound treatment.
Keywords: alternative treatments; antibacterial; antibiotic resistance; honey; minimum inhibitory concentration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Magill S.S., Edwards J.R., Bamberg W., Beldavs Z.G., Dumyati G., Kainer M.A., Lynfield R., Maloney M., McAllister-Hollod L., Nadle J., et al. Multistate Point-Prevalence Survey of Health Care–Associated Infections. N. Engl. J. Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. - DOI - PMC - PubMed
-
- Weiner L.M., Webb A.K., Limbago B., Dudeck M.A., Patel J., Kallen A.J., Edwards J.R., Sievert D.M. Antimicrobial-Resistant Pathogens Associated With Healthcare-Associated Infections: Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2011–2014. Infect. Control Hosp. Epidemiol. 2016;37:1288–1301. doi: 10.1017/ice.2016.174. - DOI - PMC - PubMed
-
- Molan P. New Stratagies Combating Bacterial Infection. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2008. Honey: Antimicrobial Actions and Role in Disease Management; pp. 229–253.
-
- Tinsley J. The Production of Honey. Bee World. 1925;7:95–98. doi: 10.1080/0005772X.1925.11095874. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

