Comparison of a Blood Self-Collection System with Routine Phlebotomy for SARS-CoV-2 Antibody Testing
- PMID: 36010206
- PMCID: PMC9406345
- DOI: 10.3390/diagnostics12081857
Comparison of a Blood Self-Collection System with Routine Phlebotomy for SARS-CoV-2 Antibody Testing
Abstract
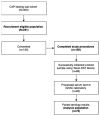
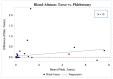
The Coronavirus Disease 2019 (COVID-19) pandemic forced researchers to reconsider in-person assessments due to transmission risk. We conducted a pilot study to evaluate the feasibility of using the Tasso-SST (Tasso, Inc, Seattle, Washington) device for blood self-collection for use in SARS-CoV-2 antibody testing in an ongoing COVID-19 prevalence and immunity research study. 100 participants were recruited between January and March 2021 from a previously identified sub-cohort of the Cabarrus County COVID-19 Prevalence and Immunity (C3PI) Study who were under-going bimonthly COVID-19 antibody testing. Participants were given a Tasso-SST kit and asked to self-collect blood during a scheduled visit where trained laboratory personnel performed routine phlebotomy. All participants completed an after-visit survey about their experience. Overall, 70.0% of participants were able to collect an adequate sample for testing using the device. Among those with an adequate sample, there was a high concordance in results between the Tasso-SST and phlebotomy blood collection methods (Cohen’s kappa coefficient = 0.88, Interclass correlation coefficient 0.98 [0.97, 0.99], p < 0.0001). The device received a high-level (90.0%) of acceptance among all participants. Overall, the Tasso-SST could prove to be a valuable tool for seroprevalence testing. However, future studies in larger, diverse populations over longer periods may provide a better understanding of device usability and acceptance among older participants and those with comorbidities in various use scenarios.
Keywords: COVID-19; SARS-CoV-2; Tasso-SST; antibody testing; capillary blood; coronavirus; infectious disease; self-collection; user acceptance.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The views expressed in this article reflect the results of the research conducted by the authors and do not reflect the official policy or position of Tasso, Inc., the Henry M. Jackson Foundation for the advancement of Military Medicine, Inc., the Department of the Navy, the Department of the Army, Department of Defense, nor the United States Government.
Figures
Similar articles
-
Comparison of capillary blood self-collection using the Tasso-SST device with venous phlebotomy for anti-SARS-CoV-2 antibody measurement.J Immunol Methods. 2023 Sep;520:113523. doi: 10.1016/j.jim.2023.113523. Epub 2023 Jul 8. J Immunol Methods. 2023. PMID: 37423588 Free PMC article.
-
Self-collection of capillary blood using Tasso-SST devices for Anti-SARS-CoV-2 IgG antibody testing.PLoS One. 2021 Sep 2;16(9):e0255841. doi: 10.1371/journal.pone.0255841. eCollection 2021. PLoS One. 2021. PMID: 34473717 Free PMC article.
-
Comparison of Capillary Blood Self-Collection using the Tasso-SST Device with Venous Phlebotomy for anti-SARS-CoV-2 Antibody Measurement.medRxiv [Preprint]. 2023 Mar 20:2023.03.13.23286935. doi: 10.1101/2023.03.13.23286935. medRxiv. 2023. Update in: J Immunol Methods. 2023 Sep;520:113523. doi: 10.1016/j.jim.2023.113523. PMID: 36993683 Free PMC article. Updated. Preprint.
-
Patient-centric microsampling for abrocitinib pharmacokinetics: multiple-analytes assay bridging using Tasso device.Bioanalysis. 2024;16(15):825-834. doi: 10.1080/17576180.2024.2388939. Epub 2024 Sep 5. Bioanalysis. 2024. PMID: 39235075 Free PMC article. Review.
-
Universal screening for SARS-CoV-2 infection: a rapid review.Cochrane Database Syst Rev. 2020 Sep 15;9(9):CD013718. doi: 10.1002/14651858.CD013718. Cochrane Database Syst Rev. 2020. PMID: 33502003 Free PMC article.
Cited by
-
Evaluating blood sampling strategies within the SIREN study: the experience from a large cohort of healthcare workers in the UK.BMC Med Res Methodol. 2025 Jul 1;25(1):165. doi: 10.1186/s12874-025-02599-x. BMC Med Res Methodol. 2025. PMID: 40596845 Free PMC article.
-
Determining the Efficacy, Feasibility, and Impact of Storage Conditions on At-Home Blood Collection Kits for Proteomic Studies.medRxiv [Preprint]. 2025 May 16:2025.05.14.25327396. doi: 10.1101/2025.05.14.25327396. medRxiv. 2025. PMID: 40463550 Free PMC article. Preprint.
-
Decentralized Clinical Trials in Early Drug Development-A Framework Proposal.J Immunother Precis Oncol. 2024 Aug 19;7(3):190-200. doi: 10.36401/JIPO-23-33. eCollection 2024 Aug. J Immunother Precis Oncol. 2024. PMID: 39219999 Free PMC article. Review.
-
Comparison of capillary blood self-collection using the Tasso-SST device with venous phlebotomy for anti-SARS-CoV-2 antibody measurement.J Immunol Methods. 2023 Sep;520:113523. doi: 10.1016/j.jim.2023.113523. Epub 2023 Jul 8. J Immunol Methods. 2023. PMID: 37423588 Free PMC article.
-
Minimally Invasive Blood Collection for an Mpox Serosurvey among People Experiencing Homelessness.J Appl Lab Med. 2024 Sep 3;9(5):1014-1019. doi: 10.1093/jalm/jfae035. J Appl Lab Med. 2024. PMID: 38842196 Free PMC article.
References
-
- Elbadawy H.M., Khattab A., Alalawi A., Dakilallah Aljohani F., Sundogji H., Mahmoud A.S., Abouzied M., Eltahir H.M., Alahmadey Z., Bahashwan S., et al. The detection of SARS-CoV-2 in outpatient clinics and public facilities during the COVID-19 pandemic. J. Med. Virol. 2021;93:2955–2961. doi: 10.1002/jmv.26819. - DOI - PMC - PubMed
-
- Jinadatha C., Jones L.D., Choi H., Chatterjee P., Hwang M., Redmond S.N., Navas M.E., Zabarsky T.F., Bhullar D., Cadnum J.L., et al. Transmission of SARS-CoV-2 in Inpatient and Outpatient Settings in a Veterans Affairs Health Care System. Open Forum Infect. Dis. 2021;8:ofab328. doi: 10.1093/ofid/ofab328. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous