Comparison of a Blood Self-Collection System with Routine Phlebotomy for SARS-CoV-2 Antibody Testing
- PMID: 36010206
- PMCID: PMC9406345
- DOI: 10.3390/diagnostics12081857
Comparison of a Blood Self-Collection System with Routine Phlebotomy for SARS-CoV-2 Antibody Testing
Abstract
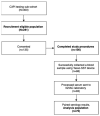
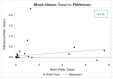
The Coronavirus Disease 2019 (COVID-19) pandemic forced researchers to reconsider in-person assessments due to transmission risk. We conducted a pilot study to evaluate the feasibility of using the Tasso-SST (Tasso, Inc, Seattle, Washington) device for blood self-collection for use in SARS-CoV-2 antibody testing in an ongoing COVID-19 prevalence and immunity research study. 100 participants were recruited between January and March 2021 from a previously identified sub-cohort of the Cabarrus County COVID-19 Prevalence and Immunity (C3PI) Study who were under-going bimonthly COVID-19 antibody testing. Participants were given a Tasso-SST kit and asked to self-collect blood during a scheduled visit where trained laboratory personnel performed routine phlebotomy. All participants completed an after-visit survey about their experience. Overall, 70.0% of participants were able to collect an adequate sample for testing using the device. Among those with an adequate sample, there was a high concordance in results between the Tasso-SST and phlebotomy blood collection methods (Cohen’s kappa coefficient = 0.88, Interclass correlation coefficient 0.98 [0.97, 0.99], p < 0.0001). The device received a high-level (90.0%) of acceptance among all participants. Overall, the Tasso-SST could prove to be a valuable tool for seroprevalence testing. However, future studies in larger, diverse populations over longer periods may provide a better understanding of device usability and acceptance among older participants and those with comorbidities in various use scenarios.
Keywords: COVID-19; SARS-CoV-2; Tasso-SST; antibody testing; capillary blood; coronavirus; infectious disease; self-collection; user acceptance.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. The views expressed in this article reflect the results of the research conducted by the authors and do not reflect the official policy or position of Tasso, Inc., the Henry M. Jackson Foundation for the advancement of Military Medicine, Inc., the Department of the Navy, the Department of the Army, Department of Defense, nor the United States Government.
Figures
References
-
- Elbadawy H.M., Khattab A., Alalawi A., Dakilallah Aljohani F., Sundogji H., Mahmoud A.S., Abouzied M., Eltahir H.M., Alahmadey Z., Bahashwan S., et al. The detection of SARS-CoV-2 in outpatient clinics and public facilities during the COVID-19 pandemic. J. Med. Virol. 2021;93:2955–2961. doi: 10.1002/jmv.26819. - DOI - PMC - PubMed
-
- Jinadatha C., Jones L.D., Choi H., Chatterjee P., Hwang M., Redmond S.N., Navas M.E., Zabarsky T.F., Bhullar D., Cadnum J.L., et al. Transmission of SARS-CoV-2 in Inpatient and Outpatient Settings in a Veterans Affairs Health Care System. Open Forum Infect. Dis. 2021;8:ofab328. doi: 10.1093/ofid/ofab328. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous